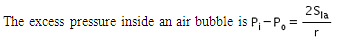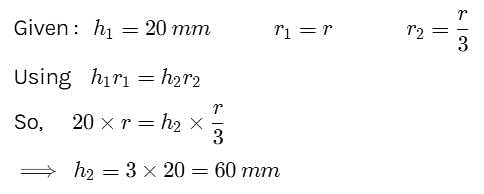Test: Surface Tension & Capillarity - NEET MCQ
10 Questions MCQ Test Physics Class 11 - Test: Surface Tension & Capillarity
The angle of contact for liquid on a solid surface is the angle between:
When impurity is added to a liquid, its surface tension
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If drops and bubbles do not collapse under the effect of gravity, it indicates that
By which phenomenon does the water rise from roots to leaves of plants?
When a capillary tube of radius r is dipped in a liquid of density ρ and surface tension S, the liquid rises or falls through a distance
When an air bubble of radius R lies at a depth h below the free surface of a liquid of density ρ and surface tension Sla, then the excess pressure inside the bubble will be
Water rises to a height of 20 mm in a capillary. If the radius of the capillary is made 1/3 rd of its previous value, to what height will the water now rise in the tube?
The excess pressure inside a soap bubble is (Here, Sla is the surface tension between the liquid-air interface).
|
98 videos|388 docs|105 tests
|