Case Based Questions Test: Thermal Properties of Matter - NEET MCQ
10 Questions MCQ Test Topic-wise MCQ Tests for NEET - Case Based Questions Test: Thermal Properties of Matter
Attempt All sub parts from each question.
Triple Point: The temperature of a substance remains constant during its change of state (phase change). A graph between the temperature T and the Pressure P of the substance is called a phase diagram or P-T diagram. The following figure shows the phase diagram of water and CO2. Such a phase diagram divides the P-T plane into a solid-region, the vapour-region and the liquid-region. The regions are separated by the curves such as sublimation curve (BO), fusion curve (AO) and vaporisation curve (CO). The points on sublimation curve represent states in which solid and vapour phases coexist. The point on the sublimation curve BO represent states in which the solid and vapour phases coexist. Points on the fusion curve AO represent states in which solid and liquid phase coexist. Points on the vaporisation curve CO represent states in which the liquid and vapour phases coexist. The temperature and pressure at which the fusion curve, the vaporisation curve and the sublimation curve meet and all the three phases of a substance coexist is called the triple point of the substance.
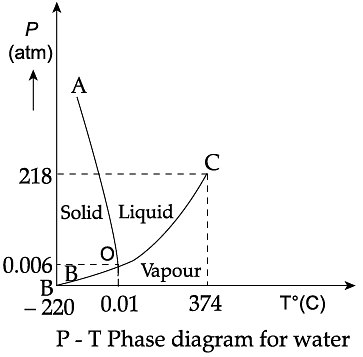
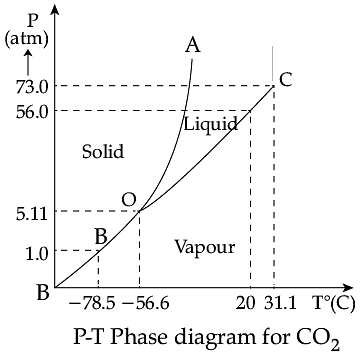
Q. Sublimation curve represents the coexistence of


Attempt All sub parts from each question.
Triple Point: The temperature of a substance remains constant during its change of state (phase change). A graph between the temperature T and the Pressure P of the substance is called a phase diagram or P-T diagram. The following figure shows the phase diagram of water and CO2. Such a phase diagram divides the P-T plane into a solid-region, the vapour-region and the liquid-region. The regions are separated by the curves such as sublimation curve (BO), fusion curve (AO) and vaporisation curve (CO). The points on sublimation curve represent states in which solid and vapour phases coexist. The point on the sublimation curve BO represent states in which the solid and vapour phases coexist. Points on the fusion curve AO represent states in which solid and liquid phase coexist. Points on the vaporisation curve CO represent states in which the liquid and vapour phases coexist. The temperature and pressure at which the fusion curve, the vaporisation curve and the sublimation curve meet and all the three phases of a substance coexist is called the triple point of the substance.
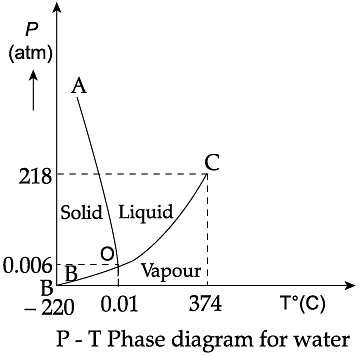
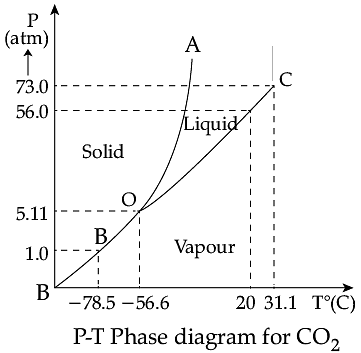
Q. During phase change of a substance


| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Attempt All sub parts from each question.
Triple Point: The temperature of a substance remains constant during its change of state (phase change). A graph between the temperature T and the Pressure P of the substance is called a phase diagram or P-T diagram. The following figure shows the phase diagram of water and CO2. Such a phase diagram divides the P-T plane into a solid-region, the vapour-region and the liquid-region. The regions are separated by the curves such as sublimation curve (BO), fusion curve (AO) and vaporisation curve (CO). The points on sublimation curve represent states in which solid and vapour phases coexist. The point on the sublimation curve BO represent states in which the solid and vapour phases coexist. Points on the fusion curve AO represent states in which solid and liquid phase coexist. Points on the vaporisation curve CO represent states in which the liquid and vapour phases coexist. The temperature and pressure at which the fusion curve, the vaporisation curve and the sublimation curve meet and all the three phases of a substance coexist is called the triple point of the substance.
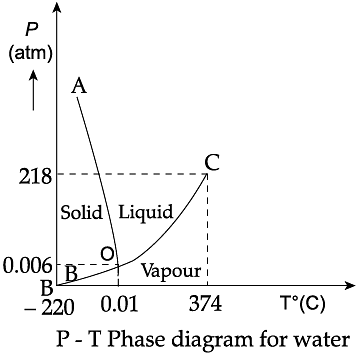
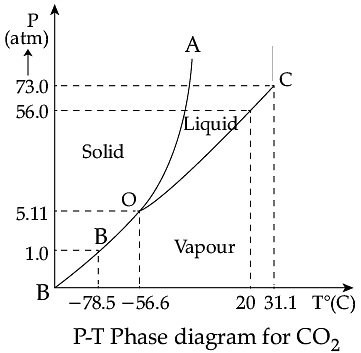
Q. Triple point of CO2 is


Attempt All sub parts from each question.
Triple Point: The temperature of a substance remains constant during its change of state (phase change). A graph between the temperature T and the Pressure P of the substance is called a phase diagram or P-T diagram. The following figure shows the phase diagram of water and CO2. Such a phase diagram divides the P-T plane into a solid-region, the vapour-region and the liquid-region. The regions are separated by the curves such as sublimation curve (BO), fusion curve (AO) and vaporisation curve (CO). The points on sublimation curve represent states in which solid and vapour phases coexist. The point on the sublimation curve BO represent states in which the solid and vapour phases coexist. Points on the fusion curve AO represent states in which solid and liquid phase coexist. Points on the vaporisation curve CO represent states in which the liquid and vapour phases coexist. The temperature and pressure at which the fusion curve, the vaporisation curve and the sublimation curve meet and all the three phases of a substance coexist is called the triple point of the substance.


Attempt All sub parts from each question.
Triple Point: The temperature of a substance remains constant during its change of state (phase change). A graph between the temperature T and the Pressure P of the substance is called a phase diagram or P-T diagram. The following figure shows the phase diagram of water and CO2. Such a phase diagram divides the P-T plane into a solid-region, the vapour-region and the liquid-region. The regions are separated by the curves such as sublimation curve (BO), fusion curve (AO) and vaporisation curve (CO). The points on sublimation curve represent states in which solid and vapour phases coexist. The point on the sublimation curve BO represent states in which the solid and vapour phases coexist. Points on the fusion curve AO represent states in which solid and liquid phase coexist. Points on the vaporisation curve CO represent states in which the liquid and vapour phases coexist. The temperature and pressure at which the fusion curve, the vaporisation curve and the sublimation curve meet and all the three phases of a substance coexist is called the triple point of the substance.
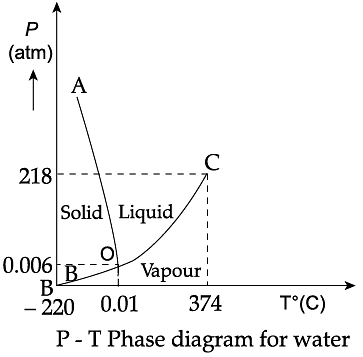
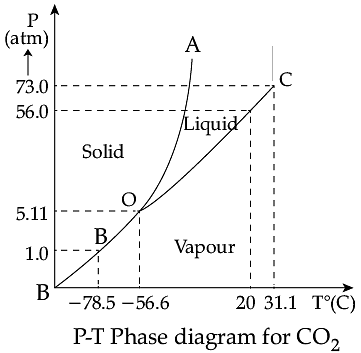
Q. The temperature and pressure at which all the three phases of a substance coexist is called
Attempt All sub parts from each question.
Railway track expansion joint: Expansion and contraction of steel and concrete structure due to seasonal heating and cooling is a common problem found in civil engineering. To combat this problem, engineers put expansion joints to absorb these changes.

This problem is compounded on railway tracks. This could lead to rail buckling, known in the industry as “sun kink”, as shown below, and cause the derailment of train. When exposed to temperature variations, the rail tends to vary its length. If this tendency is freely allowed, for a temperature

variance Δt, the rail length L will vary by ΔL. This length variance can be computed as:
ΔL = αLΔt
where α = expansion coefficient of steel
= 11.5 x 10–6 °C.
The coefficient of thermal expansion is defined as the fractional increase in length per unit rise in temperature. Traditional railway tracks are of standard lengths. When the tracks are laid, the lengths are joined end to end using “fishplates”—short lengths of steel plate overlapping the joint, and bolted to the ends of the rails. At each joint there must be a short gap (≈ 1/8") between the rail ends, to allow for longitudinal thermal expansion of the rails on hot days.
Q. “Sun kink” is the
Attempt All sub parts from each question.
Railway track expansion joint: Expansion and contraction of steel and concrete structure due to seasonal heating and cooling is a common problem found in civil engineering. To combat this problem, engineers put expansion joints to absorb these changes.

This problem is compounded on railway tracks. This could lead to rail buckling, known in the industry as “sun kink”, as shown below, and cause the derailment of train. When exposed to temperature variations, the rail tends to vary its length. If this tendency is freely allowed, for a temperature

variance Δt, the rail length L will vary by ΔL. This length variance can be computed as:
ΔL = αLΔt
where α = expansion coefficient of steel
= 11.5 x 10–6 °C.
The coefficient of thermal expansion is defined as the fractional increase in length per unit rise in temperature. Traditional railway tracks are of standard lengths. When the tracks are laid, the lengths are joined end to end using “fishplates”—short lengths of steel plate overlapping the joint, and bolted to the ends of the rails. At each joint there must be a short gap (≈ 1/8") between the rail ends, to allow for longitudinal thermal expansion of the rails on hot days.
Q. What will be the expansion of a 20m long railway steel track for 30°C variation of temperature?
Attempt All sub parts from each question.
Railway track expansion joint: Expansion and contraction of steel and concrete structure due to seasonal heating and cooling is a common problem found in civil engineering. To combat this problem, engineers put expansion joints to absorb these changes.

This problem is compounded on railway tracks. This could lead to rail buckling, known in the industry as “sun kink”, as shown below, and cause the derailment of train. When exposed to temperature variations, the rail tends to vary its length. If this tendency is freely allowed, for a temperature

variance Δt, the rail length L will vary by ΔL. This length variance can be computed as:
ΔL = αLΔt
where α = expansion coefficient of steel
= 11.5 x 10–6 °C.
The coefficient of thermal expansion is defined as the fractional increase in length per unit rise in temperature. Traditional railway tracks are of standard lengths. When the tracks are laid, the lengths are joined end to end using “fishplates”—short lengths of steel plate overlapping the joint, and bolted to the ends of the rails. At each joint there must be a short gap (≈ 1/8") between the rail ends, to allow for longitudinal thermal expansion of the rails on hot days.
Q. Which of the following statement is true?
Attempt All sub parts from each question.
Railway track expansion joint: Expansion and contraction of steel and concrete structure due to seasonal heating and cooling is a common problem found in civil engineering. To combat this problem, engineers put expansion joints to absorb these changes.

This problem is compounded on railway tracks. This could lead to rail buckling, known in the industry as “sun kink”, as shown below, and cause the derailment of train. When exposed to temperature variations, the rail tends to vary its length. If this tendency is freely allowed, for a temperature

variance Δt, the rail length L will vary by ΔL. This length variance can be computed as:
ΔL = αLΔt
where α = expansion coefficient of steel
= 11.5 x 10–6 °C.
The coefficient of thermal expansion is defined as the fractional increase in length per unit rise in temperature. Traditional railway tracks are of standard lengths. When the tracks are laid, the lengths are joined end to end using “fishplates”—short lengths of steel plate overlapping the joint, and bolted to the ends of the rails. At each joint there must be a short gap (≈ 1/8") between the rail ends, to allow for longitudinal thermal expansion of the rails on hot days.
Q. At each railway track joint a short gap of ............... (approximately) is left.
Attempt All sub parts from each question.
Railway track expansion joint: Expansion and contraction of steel and concrete structure due to seasonal heating and cooling is a common problem found in civil engineering. To combat this problem, engineers put expansion joints to absorb these changes.

This problem is compounded on railway tracks. This could lead to rail buckling, known in the industry as “sun kink”, as shown below, and cause the derailment of train. When exposed to temperature variations, the rail tends to vary its length. If this tendency is freely allowed, for a temperature

variance Δt, the rail length L will vary by ΔL. This length variance can be computed as:
ΔL = αLΔt
where α = expansion coefficient of steel
= 11.5 x 10–6 °C.
The coefficient of thermal expansion is defined as the fractional increase in length per unit rise in temperature. Traditional railway tracks are of standard lengths. When the tracks are laid, the lengths are joined end to end using “fishplates”—short lengths of steel plate overlapping the joint, and bolted to the ends of the rails. At each joint there must be a short gap (≈ 1/8") between the rail ends, to allow for longitudinal thermal expansion of the rails on hot days.
Q. What is the value of thermal expansion coefficient of steel?
|
9 docs|1272 tests
|

















