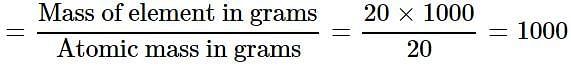Test: Mole Concept & Molar Mass (April 19) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Mole Concept & Molar Mass (April 19)
Match the column I with column II and mark the appropriate choice.


The density of a gas is 1.78 g L-1 at STP. The weight of one mole of gas is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
How many number of molecules and atoms respectively are present in 2.8 litres of a diatomic gas at STP?
How many atoms in total are present in 1 kg of sugar?
What will be the standard molar volume of He, if its density is 0.1784 g/L at STP?
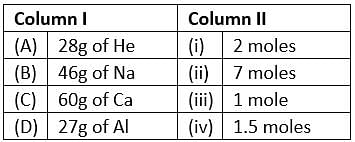
Match the mass of elements given in column I with the no. of moles given in column II and mark the appropriate choice.
Which of the following correctly represents 180 g of water?
(i) 5 moles of water
(ii) 10 moles of water
(iii) 6.023 x 1023 molecules ofwater
(iv) 6.023 x 1024 molecules of water
How many oxygen atoms will be present in 88 g of CO2?
One atom of an element weighs 3.32 x 10-23 g. How many number of gram atoms are there in 20 kg of the element?
The mass of one mole of a substance in grams is called its
|
12 docs|366 tests
|


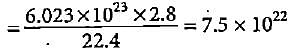 molecules
molecules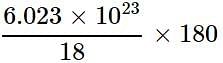 = 6.023 x 1024 molecules of H2O
= 6.023 x 1024 molecules of H2O