Chemistry: CUET Mock Test - 1 - CUET MCQ
30 Questions MCQ Test CUET Mock Test Series - Chemistry: CUET Mock Test - 1
Which of the following is regarded as the ‘repeatable entity’ of a 3D crystal structure?
In primitive unit cubic cell, only _______ of an atom (or ion or molecule) belongs to a particular unit cell.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What kind of order is present in a solid for it to be a perfectly crystalline solid?
Which of the following statements regarding Ideal solutions is false?
Which of the following unit cells has constituent particles occupying the corner positions only?
The total number of atoms in one unit cell of primitive unit cubic cell is ______ atom(s).
Which of the following can be used to describe a crystalline solid?
Which of the following is not an example of an Ideal solution?
What is the coordination number of a body-centered unit cell?
The total number of atoms in one unit cell of body-centered unit cubic cell is ______ atoms.
In a crystal, if a fault exists in the arrangement at a point, it is called as _______
Which of the following arrangements of particles does a simple cubic lattice follow?
The total number of atoms in one unit cell of face-centered unit cubic cell is ______ atoms.
In which type of point defect are the cations and anions absent in stoichiometric proportions?
Which of the following is false regarding Non-Ideal solutions?
If a crystal lattice has 6 closed-pack spheres, what the number of tetrahedral voids in the lattice?
Schottky defects are observed in solids with cations and anions of similar sizes. Which of the following compounds, therefore, is NOT likely to have a Schottky defect?
Which of the following is an example of a non-ideal solution showing positive deviation?
Which of the following possess anisotropic nature within their structure?
Impurity defect is a type of point defect. It can occur ________
Which type of solid structure melts at a definite, sharp melting point?
Identify the dimensional relation for the unit cell illustrated below.

A compound is formed by atoms of elements A occupying the corners of the unit cell and an atom of element B present at the center of the unit cell. Deduce the formula of the compound.
Which of the following is not an example of a non-ideal solution showing negative deviation?
Atoms of element X form a BCC and atoms of element Y occupy 3/4th of the tetrahedral voids. What is the formula of the compound?
Which of the following is true regarding non-ideal solutions with negative deviation?
What is the total volume of the particles present in a body centered unit cell?
|
8 docs|148 tests
|





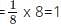
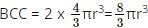 since a BCC has 2 particles per cell.
since a BCC has 2 particles per cell.














