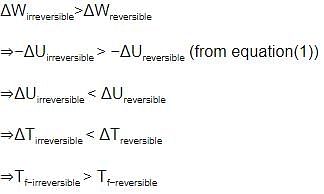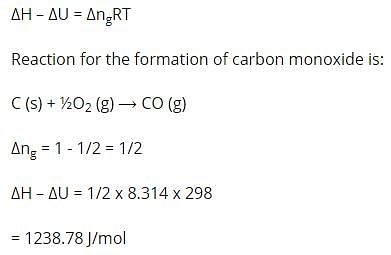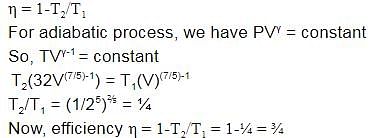Test: JEE Previous Year Questions- Thermodynamics - JEE MCQ
30 Questions MCQ Test Chapter-wise Tests for JEE Main & Advanced - Test: JEE Previous Year Questions- Thermodynamics
The quantity required to increase the temperature of a body by 1 Kelvin is called
[AIEEE-2002]
Even Carnot engine cannot give 100% efficiency because we cannot –
[AIEEE-2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which statement is incorrect ?
[AIEEE-2002]
"Heat cannot be itself flow from a body at lower temperature to a body at higher temperature" is a statement or consequence of –
[AIEEE - 2003]
Which of the following parameters does not characterize the thermodynamic state of matter ?
[AIEEE-2003]
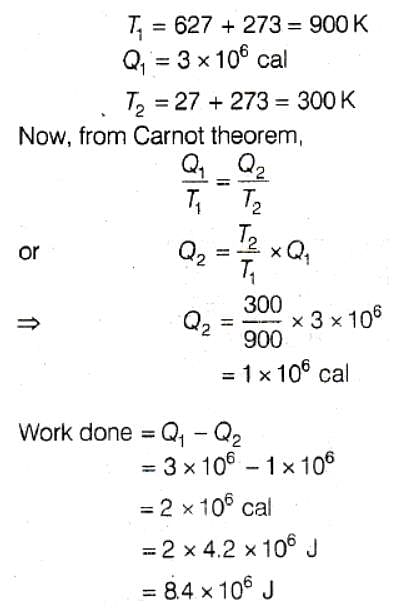
A Carnot engine takes 3 × 106 cal. of heat from a reservoir at 627ºC, and gives it to a sink at 27º C. The work done by the engine is –
[AIEEE-2003]
In an irreversible process taking place at constant T and P and in which only pressure-volume work is being done the change in Gibbs free energy (dG) and change in entropy (dS) satisfy the criteria -
[AIEEE-2003]
The internal energy change when a system goes from state A to B is 40 kJ/mole. If the system goes from A to B by a reversible path and returns to state A by an irreversible path what would be the net change in internal energy
[AIEEE-2003]
The correct relationship between free energy change in a reaction and the corresponding equilibrium constant KC is –
[AIEEE-2003]
Which of the following statements is correct for any thermodynamic system ?
[AIEEE-2004]
An ideal gas expands in volume from 1 × 10-3 m3 to 1 × 10-2 m3 at 300 K against a constant pressure of 1 × 105 Nm-2. The work done is -
[AIEEE-2004]
Which of the following is incorrect regarding the first law of thermodynamics ?
[AIEEE-2005]
The temperature-entropy diagram of a reversible engine cycle is given in the figure. Its efficiency is -
[AIEEE-2005]
A system goes from A to B via two processes I and II as shown in figure. If ΔU1 and ΔU2 are the changes in internal energies in the processes I and II respectively, then
[AIEEE-2005]
Consider the reaction : N2 + 3 H2 → 2 NH3 carried out at constant temperature and pressure. If ΔH and ΔU are the enthalpy and internal energy changes for the reaction, which of the following expressions is true ?
[AIEEE-2005]
The work of 146 kJ is performed in order to compress one kilo mole of a gas adiabatically and in this process the temperature of the gas increases by 7º C. The gas is - (R = 8.3 J mol-1 K-1) –
[AIEEE 2006]
An ideal gas is allowed to expand both reversibly and irreversibly in an isolated system. If Ti is the initial temperature and Tf is the final temperature, which of the following statements is correct
[AIEEE 2006]
(ΔH - ΔH) for the formation of carbon monoxide (CO) from its elements at 298 K is (R = 8.314 J K-1 mol-1)–
[AIEEE 2006]
A Carnot engine, having an efficiency of h = 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
[AIEEE 2007]
When a system is taken from state i to state f along the path iaf, it is found that Q = 50 cal and W = 20 cal. Along the path ibf Q= 36 cal. W along the path ibf is -
[AIEEE 2007]
In conversion of lime-stone to lime, CaCO3(s) → CaO(s) + CO2(g) the values of ΔHº and ΔSº are + 179.1 kJ mol-1 and 160.2 J/K respectively at 298K and 1 bar. Assuming that ΔHº and ΔSº do not change with temperature, temperature above which conversion of limestone to lime will be spontaneous is-
[ AIEEE 2007]
Assuming that water vapour is an ideal gas, the internal energy change(ΔU) when 1 mol of water is vapourised at 1 bar pressure and 100°C, (Given : Molar enthalpy of vapourisation of water at 1 bar and 373 K = 41 kJ mol-1 and R = 8.3 J mol-1K-1 ) will be -
[AIEEE 2007]
Identify the correct statement regarding a spontaneous process –
[AIEEE 2007]
Standard entropy of X2, Y2 and XY3 are 60, 40 and 50 J K-1 mol-1, respectively. For the reaction, X2 +
Y2 – XY3 DH = – 30 kJ, to be at equilibrium, the temperature will b[AIEEE 2008]
In a fuel cell methanol is used as fuel and oxygen gas is used as an oxidizer. The reaction is CH3OH(l) + O2(g) → CO2(g) + 2H2O(l) At 298 K standard Gibb's energies of formation for CH3OH(l), H2O(l) and CO2(g) are –166.2, –237.2 and –394.4 kJ mol-1 respectively. If standard enthalpy of combustion of methanol is –726 kJ mol-1, efficiency of the fuel cell will be-
[AIEEE 2009]
A diatomic ideal gas is used in a Carnot engine as the working substance. If during the adiabatic expansion part of the cycle the volume of the gas increases from V to 32 V, the efficiency of the engine is-
For a particular reversible reaction at temperature T, ΔH and ΔS were found to be both +ve. If is the temperature at equilibrium, the reaction would be spontaneous when
[AIEEE 2010]
The entropy change involved in the isothermal reversible expansion of 2 moles of an ideal gas from a volume of 10 dm3 to a volume of 100 dm3 at 27º C is :
[AIEEE 2011]
In view of the signs of DrG° for the following reactions :
PbO2 + Pb → 2 PbO, DrG° < 0
SnO2 + Sn → 2 SnO, DrG° > 0,
which oxidation states are more characteristic for lead and tin ? [AIEEE 2011]
The incorrect expression among the following is –
[AIEEE-2012]
|
446 docs|930 tests
|
|
446 docs|930 tests
|