(03-01-2018) Mole Concept, Gaseous & Surface Chemistry - IIT JAM MCQ
30 Questions MCQ Test - (03-01-2018) Mole Concept, Gaseous & Surface Chemistry
Arrange the following gases in the increasing order of ‘b’. O2, CO2, H2, He:
Gases possess characteristic critical temperature which depends upon the magnitude of intermolecular forces between the particles.
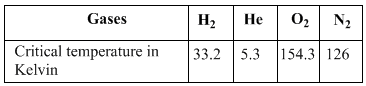
What would be the order of liquefaction of these gases? Start writing the order from the gas liquefying first.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The experimental adsorption data of a gas on a solid surface at temperature T exhibits the following variation with pressure. V is the volume of gas adsorbed.
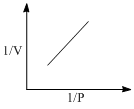
Which of the following is true?

The molar heat capacity at constant volume of a colourless gas is found to be 25 J mol–1 K–1 at room temperature. The gas must be:
Number of atoms in the following samples of substances is the largest in:
How many mole of electrons are required to reduce 1.5 mole of nitro-benzene into aniline, if current efficiency, in this experiment is 50%:
How many moles of KMnO4 would be required to oxidize a mixture of FeC2O4, FeSO4, Fe2(SO4)3 and Fe2(C2O4)3 which contains 1 mole each of these compounds in an acidic medium:
CH3COOH exists as dimer in benzene, 1.2 gm of the acid was dissolved and the volume was made up tone litre by benzene, what is the formality of the acid:
5 g of a metal gave 6.35 gm of its oxide. Calculate the equivalent weight of metal:
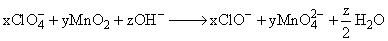
In this balanced equation x, y, z are:
At 298 K equal weights of SO2, CH4 and O2 are mixed in empty container. The total pressure exerted is 2.1 atm. The partial pressure of CH4 in the mixture is:
Once Tom & Jerry entered a chemistry lab where a chemist was preparing 3L H2SO4 solution. He labeled the solution as 10 m (dsolution = 3.3 gm/ml). As the chemist left the lab, a mischief came in Tom’s mind. He tried to throw the solution on Jerry but failed. In doing so some of the H2SO4 solution fell on the floor, so he added water to make it again to 3L. The chemist returned back and got astonished when he saw the result of analysis, that were d = 1.5 gm/ml and % w/w = 98. Find out the moles of H2SO4 which fell down on the floor.
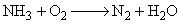
If molecular weights of NH3 and N2 are M1 and M2 respectively, and their equivalent weights are E1 and E2 respectively, then (M1 – M2) is equal to:
A mixture of H2SO4 and H2C2O4 (oxalic acid) and some inert impurity weighing 3.185 g was dissolved in water and the solution made up to 1 litre. 10 mL of this solution required 3 mL of 0.1 N NaOH for complete neutralization. In another experiment, 100 mL of the same solution in hot condition required 4 mL of 0.02 M KMnO4 solution for complete reaction. The % by weight of H2SO4 in the mixture was:
A flask of 2.05 litre volume contains pure F2 (gas) at 4 atm and 2000 K. To this flask, some solid sulphur is added from outside and sealed. Fluorine reacts completely to form a gaseous fluoride and pressure decreases to 2 atm. Assuming temperature to be constant, the formula of fluoride will be
(R = 0.082 litre atm/mol/k)
When an equimolar mixture of Cu2S and CuS is titrated with Ba(MnO4)2 in acidic medium, the final product contains Cu+2, SO2 and Mn+2. If the molecular weight of Cu2S, CuS and Ba(MnO4)2 are M1, M2 and M3 respectively then:
An atom of element ‘X’ is 1.02 times heavier than that of an atom of ‘Y’. An atom of ‘Y’ is 0.1809 times heavier than that of an atom of oxygen. What is the atomic weight of ‘X’:
Two flasks A and B of equal volume containing NH3 and HCl gases, are connected by a narrow tube to negligible volume. The two gases were prevented from mixing by stopper fitted in connecting tube. For further detail of experiment refer to the given figure. What will be final pressure in each flask when passage connecting two tubes are opened. Assume ideal gas behaviour of NH3 and HCl gas and the reaction.
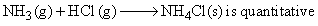
The mean free path of gas A, with molecular diameter equal to 4 Å, contained in a vessel, at a pressure of 10–6 torr, is 6990 cm. The vessel is evacuated and then filled with gas B, with molecular diameter, equal to 2 Å, at a pressure of 10–3 torr, the temperature remaining the same. The mean free path of gas B will be:
If the slope of Z (compressibility factor) v/s p curve is constant 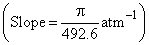 at a particular temperature (300 K) and very high pressure, then calculate diameter of the molecules
at a particular temperature (300 K) and very high pressure, then calculate diameter of the molecules
(Given NAV = 6 × 1023, R = 0.081 L atm K–1 mol–1)
A mixture weighing 4.08 g of BaO and unknown carbonate XCO3 was heated strongly. The residue weighed 3.64 g. This was dissolved in 100 mL of 1 N HCl. The excess acid required 16 mL of 2.5 N NaOH solution for complete neutralization. Identify the metal M:
13.8 g of Ag2CO3 is strongly heated. If released gases are used for combustion of C2H2, the number of moles of C2H2 oxidized are:
Assume that you are using an open-end manometer filled with mineral oil rather than mercury. What is the gas pressure in the bulb (in millimeter of Hg) if the level of mineral oil in the arm connected to the bulb is 237 mm higher than the level in the arm connected to the atmosphere and atmospheric pressure is 746 mm Hg? The density of mercury is 13.6 g/ml. And density of mineral oil is 0.822 g/mol.
Graph between 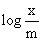 and log P is a straight line inclined at an angle q = 45°. When pressure of 0.5 atm and log k = 0.699, the amount of solute adsorbed per g of adsorbent will be:
and log P is a straight line inclined at an angle q = 45°. When pressure of 0.5 atm and log k = 0.699, the amount of solute adsorbed per g of adsorbent will be:
The Langmuir adsorption isotherm is given by 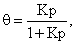 where P is the pressure of the adsorbate gas. The Langmuir adsorption isotherm for a diatomic gas A2 undergoing dissociative adsorption is:
where P is the pressure of the adsorbate gas. The Langmuir adsorption isotherm for a diatomic gas A2 undergoing dissociative adsorption is:
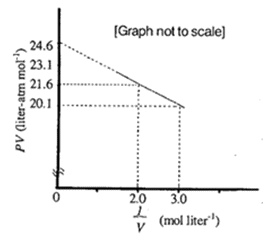
For one mole of a van der Waal’s gas when b = 0 and T = 300 K, the pV vs. 1/V plot is shown below. The value of the van der Waal’s constant a (atm L mol–2)
N moles of diatomic gas in a cylinder are at a temperature T. Heat is supplied to the cylinder such that the temperature remains constant but n moles of the diatomic gas get converted into monoatomic gas. What is the change in the total kinetic energy of the gas?
At which location in the inside surface of the closed container shown in figure will the number of gaseous molecular collisions per unit area be the greatest? (Ignore the effects of gravity.)
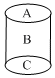
Consider the composite system, which is held at 300 K, shown in below figure. Assuming ideal gas behaviour, calculate the total pressure if the barriers separating the compartments are removed. Assuming that the volume of the barriers is negligible. (Given: R = 0.082 L atm mol–1 K–1)

3 g of activated charcoal wad added to 50 mL of acetic acid solution (0.06 N) in a flask. After an hour it was filtered and the strength of the filtrate was found to be 0.042 N. The amount of acetic acid adsorbed (per gram of charcoal) is:

















