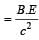31 Year NEET Previous Year Questions: Nuclei - 2 - NEET MCQ
30 Questions MCQ Test - 31 Year NEET Previous Year Questions: Nuclei - 2
The activity of a radioactive sample is measuredas 9750 counts per minute at t = 0 and as 975counts per minute at t = 5 minutes. The decayconstant is approximately [1997]
The stable nucleus that has a radius half that of Fe56 is [1997]
In a fission reaction
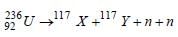
the binding energy per nucleon of X and Y is 8.5MeV whereas of 236U is 7.6 MeV. The totalenergy liberated will be about [1997]
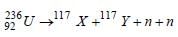
A free neutron decays into a proton, an electronand [1997]
The most penetrating radiation of the following is [1997]
Which of the following is used as a moderator innuclear reactors? [1997]
Half-lives of two radioactive substances A and B are respectively 20 minutes and 40 minutes.Initially, the samples of A and B have equalnumber of nuclei. After 80 minutes the ratio ofremaining numbers of A and B nuclei is [1998]
Atomic weight of Boron is 10.81 and it has two isotopes 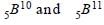 . Then the ratio
. Then the ratio 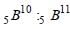 in nature would be [1998]
in nature would be [1998]
A nucleus nXm emits one α and two β particles. The resulting nucleus is
Complete the equation for the following fission process : 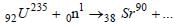 [1998]
[1998]
After 1α and 2 β-emissions [1999]
The decay constant (λ) and the half-life (T) of a radioactive isotope are related as [2000]
Atomic hydrogen has life period of [2000]
It is possible to understand nuclear fission onthe basis of the [2000]
In the following nuclear reaction
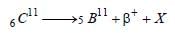
what does X stand for? [2000]
Mn and Mp represent mass of neutron and protonrespectively. If an element having atomic massM has N-neutron and Z-proton, then the correctrelation will be [2001]
A sample has 4 × 1016 radioactive nuclei of halflife 10 days. The number of atoms decaying in 30 days is [2002]
A deuteron strikes 8O16 nucleus with subsequent emission of an alpha particle. Identify the nucleus so produced [2002]
For a nuclear fusion process, the suitable nucleiare [2002]
Solar energy is mainly caused due to [2003]
A nuclear reaction is given by 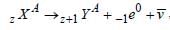 represents
represents
A sample of radioactive element has a mass of 10gm at an instant t=0. The approximate mass ofthis element in the sample after two mean lives is [2003]
The mass number of a nucleus is [2003]
The mass of proton is 1.0073 u and that ofneutron is 1.0087 u (u = atomic mass unit). Thebinding energy of 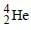 is [2003]
is [2003]
The volume occupied by an atom is greater thanthe volume of the nucleus by a factor of about[2003]
If in nuclear fusion process the masses of thefusing nuclei be m1 and m2 and the mass of theresultant nucleus be m3, then [2004]
A nucleus represented by the symbol 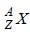 has
has
Mp denotes the mass of a proton and Mn that ofa neutron. A given nucleus, of binding energyB, contains Z protons and N neutrons. The mass M (N, Z) of the nucleus is given by (c is thevelocity of light) [2004]
The nuclei of which one of the following pairs of nuclei are isotones? [2005]


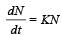
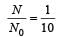
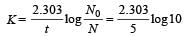
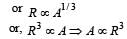
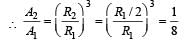
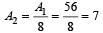

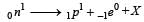
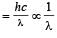
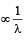
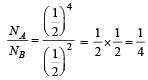
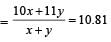
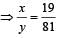
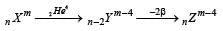
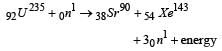
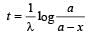
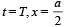
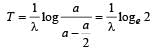
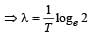
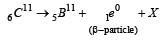
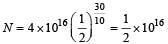
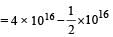

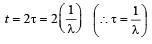
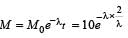
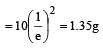
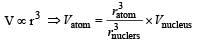
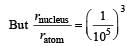
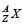 has Z protons and (A – Z) neutrons
has Z protons and (A – Z) neutrons