AFMC Chemistry Mock Test - 1 - NEET MCQ
30 Questions MCQ Test AFMC Mock Test Series 2025 - AFMC Chemistry Mock Test - 1
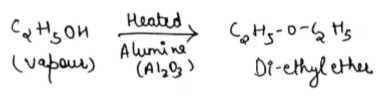
Which of the following compound is obtained on passing ethanol vapours on heated Al₂O₃?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following cannot reduce Fehling solution?
The atomic numbers of elements X, Y, and Z are 19, 21, and 25 respectively. The number of electrons present in the M-shells of these elements follow the order.
Which of the followng acts to inhibit lutenizing hormone secretion?
Which one of the following is the correct set with reference to molecular formula, hybridisation of central atom and shape of the molecule
If a mixture containing 3 moles of hydrogen and 1 mole of nitrogen is converted completely into ammonia, the ratio of volumes of reactants and products at the same temperature and pressure would be
The facor which changes equilibrium constant of the reaction A₂(g) + B₂(g) → 2AB(g) is
The activation energy of a chemical reaction can be determined by
The first ionisation potentials of four consecutive elements, present in the second period of the periodic table are 8.3, 11.3, 14.5 and 13.6 eV respectively. Which one of the following is the first ionisation potential (in eV) of nitrogen?
Which of the following endothermic process is spontaneous?
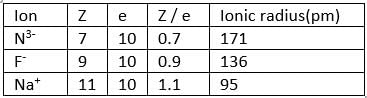
Identify the correct order in which the ionic radius of the following ions increases:
I) F −
II) Na+
III) N3 −
The synthetic product which closely resembles with the natural musk in odour is
In which of the following reaction, hydrogen is acting as an oxidising agent?
Which of the following alkanes has neither secondary nor tertiary hydrogens ?
Presence of which of the following salts increases the rate of setting of plaster of paris.
An aqueous solution of sodium carbonate is alkaline because sodium carbonate is a salt of
Lithium and magnesium are similar in their properties due to the same
Which of the following isomers will have the highest boiling point ?
The solubility of AgI in NaI solution is less than that in pure water because
The half life period of a radioactive material is 15 minutes. What percent of radioactivity of that material will remain after 45 minutes?
|
1 docs|30 tests
|