AIIMS Full Mock Test 18 - NEET MCQ
30 Questions MCQ Test AIIMS Mock Tests & Previous Year Question Papers - AIIMS Full Mock Test 18
In a pure silicon (ni = 1016/m3) crystal at 300 K, 1021 atoms of phosphorus are added per cubic meter. The new hole concentration will be
In transistor, forward bias is always smaller than the reverse bias. The correct reason is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
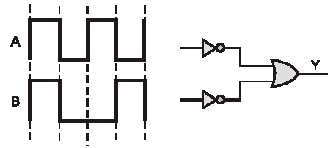
In a given circuit as shown the two input waveform A and B are applied simultaneously. The resultant waveform Y is
In the following circuit, a voltmeter V is connected across a lamp L. What change would occur in voltmeter reading if the resistance R is reduced in value.
In the study of transistor as an amplifier, if a = Ic / Ie and b = Ic / Ib, where Ic ,Ib and Ie are the collector, base and emitter currents, then
For the given combination of gates, if the logic state of inputs A, B, C are as follows A = B = C = 0 and A = B = 1, C = 0 then the logic states o output D are
Which logic gate is represented by the following combination of logic gates
In the diagram, the input is across the terminals A and C and the output is across the terminals B and D, then the output is
The phase difference between input and output voltages of a CE circuit is
The energy band diagrams for three semiconductor samples of silicon are as shown. We can then assert that
The dominant mechanisms for motion of charge carriers in forward and reverse biased silicon P-N junctions are
Consider the following statements A and B and identify the correct choice of the given answers
A: The width of the depletion layer in a P-N junction diode increases in forwards bias
B : In an intrinsic semiconductor the fermi energy level is exactly in the middle of the forbidden gap
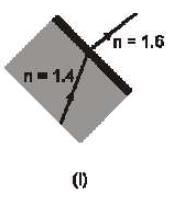
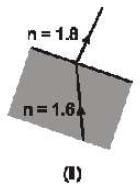
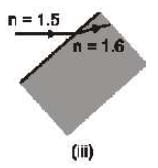
Which of the following ray diagram show physically possible refraction
When the rectangular metal tank is filled to the top with an unknown liquid, as observer with eyes level with eyes level with the top of the tank can just see the corner E; a ray that refracts towards the observer at the top surface of the liquid is shown. The refractive index of the liquid will be
PQR is a right angled prism with other angles as 60º a d 30º. Ref ractive index of prism is 1.5 PQ has a thin layer of liquid. Light falls normall on the face PR. For total internal reflection, maximum refractive index of liquid is
When a ray is refracted from one medium to another, th wavelength changes from 6000 Å to 4000 Å. The critical angle for the interface will be
Two thin lenses, when in contact, produce a combination of power + 10 D. When they are 0.25 m apart, the power reduces to + 6D. The focal lengths of the lenses (in m) are
The plane faces of two identical plane convex lances, each with focal length f are pressed against each other using an optical glue to forma usual convex lens. The distance from the optical center at which an object must be placed to obtain the image same as the size if object is
A parallel beamof light emerges fromthe opposite surface of the sphere when a point source of light lies at the surface of the sphere. The refractive index of the sphere is
A thin rod of 5 cmlength is kept along the axis of a concave mirror of 10 cm focal length such that its image is real and magnified and one end touches the rod. Its magnification will be
A lens when placed on a plane mirror then object needle and its image coincide at 15 cm. The focal length of the lens is
Flint glass prism is joined by a crown glass prism to produce dispersion without deviation. The refractive indices of these for mean rays are 1.602 and 1.500 respectively. Angle of prism of flint prism is 100, then the angle of prism for crown prism will be
An astronomical telescope has an angular magnification of magnitude 5 for distant objects. The separation between the objective and the eye-piece is 36 cm and the final image is formed at infinity. The focal length f0 of the objective and fe of the eye-piece are
The magnifying power of compound microscope in terms of the magnification m0 due to objective and magnifying power mE by the eye-piece is given by
With a simple microscope, if the final image is located at the least distance of distinct vision (i.e., D) from the eye placed close to the lens, then the magnifying power is
If in a Young's double slit experiment, the slit distance is 3 cm, the separation between slits and screen is 70cm and wavelength of light is 1000 Å , then fringe width will be
|
3 videos|44 docs|66 tests
|
|
3 videos|44 docs|66 tests
|

















