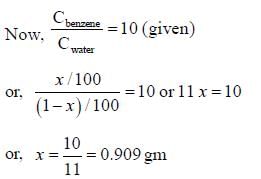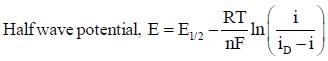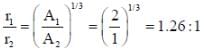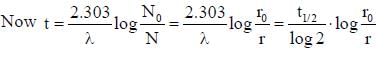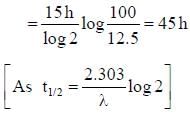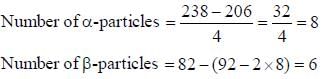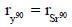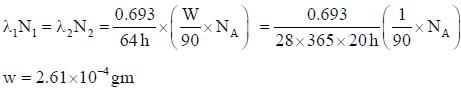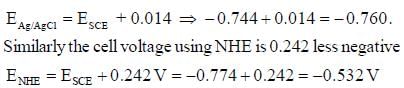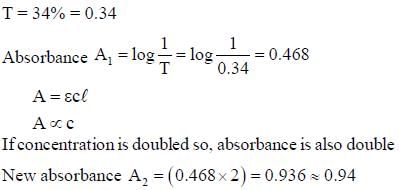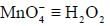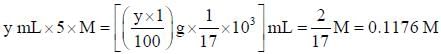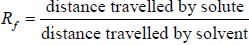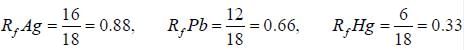Analytical & Nuclear Chemistry - GATE Chemistry MCQ
20 Questions MCQ Test GATE Chemistry Mock Test Series - Analytical & Nuclear Chemistry
The distribution coefficient of an organic compound A for benzene and water is 10. The amount of A extracted if 1.0 gm of it dissolved in 100 mL of water is equilibrated in a separatory funnel with 100 mL of benzene is ______ gm. (Round off to two one decimal place)
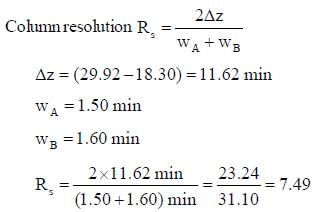
Substances X and Y were found to have a retention time of 18.30 and 29.92 minutes respectively on a 20 cm long column. The widths (at the base) for A and B were 1.50 and 1.60 minutes respectively. The column resolution is ____. (Round off to two decimal places).
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
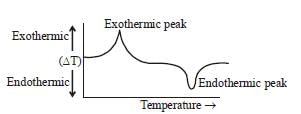
In differential thermal analysis (DTA) the correct representation of ΔT (temperature) difference between sample and thermally inert reference are continuously recorded as a function of temperature or time.
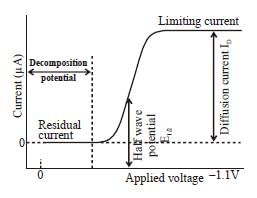
The correct form of half wave potential in a typical polarogram.
Which of the following metals can be used as indicator electrodes in potentiometric methods
Which of the following statements are correct for a successful mass spectroscopic analysis
1. Each component must exhibit atleast one peak that differs makedly from the others
2. The contribution of each component to a peak must be linearly additive
3. The sensitivity must be reproducible to 1% relative
4. Suitable standards must be available for calibration
U235 nucleus splits into two new nuclei whose mass numbers are in the ratio of 2 : 1. The ratio of the radii of the new nuclei is
Half life of Na24 radionuclide is 15h the time in which the activity of a sample of Na24 radionuclide will decrease by 87.5% is
The number of α and β particles are emitted out in the transition 92U238 to 82Pb206 are
Y90 has a half-life of 64h and Sr90 28 year. Sr90 by β-emission the amount of Y90 in equilibrium with 1 gm of Sr90 is ___ x 10-4 gm. (Round off to two decimal places)
A cell voltage measured using an SCE reference electrode is –0.774 V. (The indicates electrode is more negative half cell). The cell voltage with a Ag/AgCl reference electrode (1 MKCl : E = 0.228 V) or with NHE are
Which of the following forms of analytical chemistry seeks to obtain the condition of full polarization
Compared to AAS, AES has the advantage of providing
1.0 gram of a sample of H2O2 solution containing y% H2O2 by weight requires y mL of KMnO4 solution for complete titration under acidic condition. The molarity of KMnO4 solution is
Which type of chromatography process requires that when materials that cannot be readily dissolved in a solvent for chromatography need to be heated or pyrolyzed to a high temperature is
Differential scanning calorimetry (DSC) is a technique useful in determining
The best procedure to improve resolution between two chromatographic peak is
In paper chromatographic separation of silver, lead and Hg the solvent front was 18 cm while front due these elements were respectively 16, 12 and 6 cm. What is the Rf value of all metals.
|
18 docs|37 tests
|
|
18 docs|37 tests
|