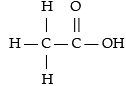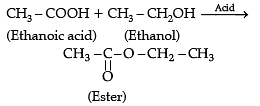Assertion & Reason Test: Carbon & Its compounds - 1 - Class 10 MCQ
10 Questions MCQ Test - Assertion & Reason Test: Carbon & Its compounds - 1
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Most of the carbon compounds are good conductors of electricity.
Reason (R): They do not dissociate to form ions and remain as molecules.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
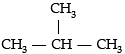
Assertion (A): Iso- butane is the isomer of C4H10.
Reason (R): Iso-butane has four C and ten-H atoms.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion: Following are the structural isomers of butane.
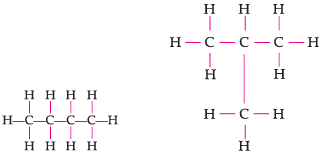
Reason: Structural isomers have the same molecular formula but they differ in their structures.

Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): In a homologous series of alcohols, the formula for the second member is C2H5OH and the third member is C3H7OH.
Reason (R): The difference between the molecular masses of the two consecutive members of a homologous series is 144u.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Third member of alkane is propane (C3H8)
Reason (R): It is obtained from general formula CnH2n + 2
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Following are the members of a homologous series : CH3OH, CH3CH2OH, CH3CH2CH2OH
Reason (R): A series of compounds with the same functional group but differing by ––CH2 – unit is called a homologous series.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): CH3Cl is obtained from CH4 by the action of Cl2 in the presence of sunlight.
Reason (R): It is obtained by an additional reaction.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): In esterification, carboxylic acid and alcohol react in the presence of acid to give ester.
Reason (R): Esterification is the reverse of saponification.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Acetic acid has six single bonds and one double bond.
Reason (R): It is an unsaturated organic compound.
Directions: In the following questions, a statement of assertion (A) is followed by a statement of reason (R). Mark the correct choice as:
Assertion (A): Esterification is a process in which a sweet smelling substance is produced.
Reason (R): When esters react with sodium hydroxide an alcohol and sodium salt of carboxylic acid are obtained.