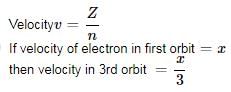Atomic Structure (Chapter Test - Non-Medical) - Class 12 MCQ
20 Questions MCQ Test - Atomic Structure (Chapter Test - Non-Medical)
The potential energy of the electron present in the ground state of Li2+ ion is represented by
If S1 be the specific charge (e/m) of cathode rays and S2 be that of positive rays then which is true ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The radii of two of the first four Bohr’s orbits of the hydrogen atom are in the ratio 1 : 4. The energy difference between them may be
The number of radial nodes of 3s and 2p-orbitals are respetively
α-Particles are ..... times heavier than neutron
An excited hydrogen atom emits a photon of wavelength λ in returning to the ground state. If R is the Rydberg constant than the quantum number n of the excited state is
A proton and an α-particle are accelerated through the same potential difference. The ratio of their de Broglie wavelength is
In an atom two electrons move around the nucleus in circular orbits of radii R and 4R. The ratio of the time taken by them to complete one revolution is
The circumference of nth orbit in H-atom can be expressed in terms of de Broglie wavelength λ as
The two particles A and B have de Broglie wavelengths 1 nm and 5 nm respectively. If mass of A is four times the mass of B, the ratio of kinetic energies would be
How fast is an electron moving, if, it has a wavelength equal to the distance it travels in 1 sec ?
One requires energy En to remove a nucleon and an energy Ee to remove an electron from the orbit of an atom, then
If for Balmer series, what is the value of c2 ?
The magnetic moment order is correctly given in
The electron identified by quantum numbers n and
I. n = 4, l = 1 II. n = 4, l = 0
III. n = 3, l = 2 IV. n = 3, l = 1
can be placed in order of increasing energy from the lowest to highest
The energy of an electron in the Bohr’s first orbit of Hatom is –13.6 eV. The possible energy value(s) of the excited state(s) for electrons in Bohr’s orbits of hydrogen is(are)
The orbital represented by ψ4,2,0 is
The angular momentum of electron in hydrogen atom is proportional to
If the speed of an electron in the Bohr’s first orbit of hydrogen atom bex , speed of the electron in 3rd orbit is
When photons of energy 4.25 eV strike the surface of a metal A, the ejected photoelectrons have maximum kinetic energy, TA (expressed in eV) and de Broglie wavelength λA, The maximum kinetic energy of photoelectrons liberated from another metal B by photons of energy 4.70 V is TB = TA –1.50eV. If the de Broglie wavelength of these photoelectrons is λB = 2λA, then which is not correct?