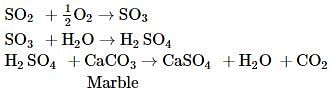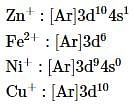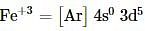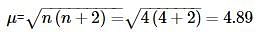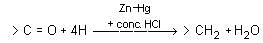BITSAT Chemistry Test - 10 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 10
Which relation is related to adsorption process, if k is the amount of adsorbent and p is the amount of adsorbate?
Identify the hybridisations of orbitals of N atom in NO3-, NO2+ and NH4sp, sp2, sp3.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following elements is/are transuranic element(s)?
(I) Uranium (Z = 92)
(II) Darmstadtium (Z = 110)
(III) Mercury (Z = 80)
(II) Darmstadtium (Z = 110)
(III) Mercury (Z = 80)
Which among the following is not a meta directing group?
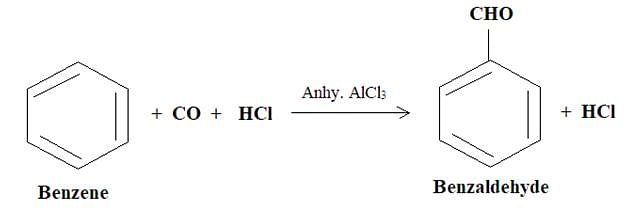
Which of the following statement(s) is true about Gattermann Koch Synthesis?
I. One of the outputs is benzaldehyde.
II. To use formyl chloride, carbon monooxide is combined with hydrochloric acid.
III. The reaction is a nucleophilic substitution reaction.
Which of the following gas is removed during the Bosch process (water gas with steam over heated catalyst)?
Which of the following electronic configurations is not correct?
An aromatic compound X when reacts with chlorine gas in the presence of iron produces chlorobenzene and when treated with concentrated H2SO4 produces benzenesulphonic acid. The compound X is likely to be
Which of the following sets represents molecules with linear geometry only?
Which of the following has the most nucleophilic nitrogen?
The primary pollutant that leads to photochemical smog is:
Which of the following gases causes greenhouse effect to the maximum extent?
Water filled in two glasses A and B gave BOD values of 10 and 20, respectively. The correct statement regarding them is
Which of the following are the hazardous pollutants present in automobile exhaust gases?
(i) N2
(ii) CO
(iii) CH4
(iv) Oxides of nitrogen
When rain is accompanied by a thunderstorm, the collected rain water has a pH Value
In any transition series, from left to right, the d -orbitals are progressively filled and their properties vary accordingly.
In the second transition series, the largest number of oxidation states are shown by
Which of the following has the highest unpaired d−electrons?
Which of the following has the electronic configuration
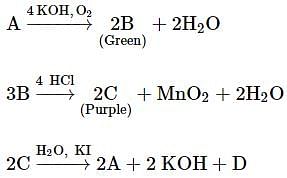
In the above sequence of reactions, A and D, respectively, are:
The spin only magnetic moment of Fe2+ ion (in BM) is approximately:
The wavelength of moving electron in 3rd Bohr's orbit of H-atom is
The proof of quantization of energy states in an atom is obtained by the experiment performed by
The number of d-electrons in Fe2+ (Z=26) is not equal to the number of electrons in which one of the following?


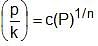

 , lone pair of nitrogen is taking part in aromaticity.
, lone pair of nitrogen is taking part in aromaticity.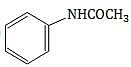 , nitrogen is attached to electron withdrawing group.
, nitrogen is attached to electron withdrawing group. , lone pair of nitrogen is in resonance with benzene ring.
, lone pair of nitrogen is in resonance with benzene ring.