BITSAT Chemistry Test - 7 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 7
Heat exchanged in a chemical reaction at the constant temperature and pressure is known as
Which of the following electronic configuration is a correct explanation of Aufbau principle ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following isomerism is exhibited byCFI3- 0 - C3H7and C2H5OC2H5?
The boiling point of three saturated hydro carbons A, Band C are - 102°C,-43.4°Cand-0.6°C respectively. The hydrocarbon having the maximum number of carbon atoms in its molecule is
The correct order of dipole moments of HF, H2S and H2O is.
Lyophillic sols are proved to be more stable than lyophobic sols, because in lyophillic sols
Tyndall effect is observed only when
(i) the diameter of the dispersed particles is not much smaller than the wavelength of the light used.
(ii) the refractive indices of dispersed phase and dispersion medium differ greatly in magnitude.
(iii) the size of the particles is generally between 10-11 and 10-9 m in diameter.
(iv) the dispersed phase and dispersion medium can be seen separately in the system.
Which property of colloidal solution is independent of charge on the colloidal particles?
Adsorption of gases on solid surface is generally exothermic because:
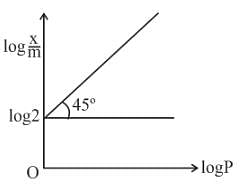
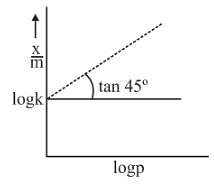
Graph between log x/m and log P is a straight line at an angle 45° with intercept is shown above x/m at a pressure of 2 atm is:
In Langmuir's model of adsorption of a gas on a solid surface
Lyophilic solutions are more stable than lyophobic solutions because
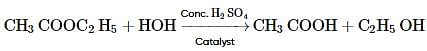
Consider the following reaction
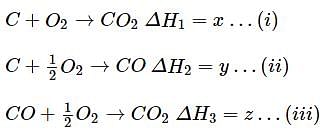
What is the relation between x,y,z
ΔG∘ for the reaction x + y ⇌ z is -4.606 kcal. The value of equilibrium constant of the reaction at 227∘ C is:
A chemical reaction will be spontaneous if it is accompanied by a decrease of
Heats of formation of SiO2 and MgO are -48.24 and -34.7 KJ/mol respectively. The heat of the reaction: 2Mg + SiO2 → 2 MgO + Si is
The values of ΔH and ΔS for the reaction, C(graphite)+CO2(g)→2CO(g) are 170 kJ and 170 JK−1, respectively. This reaction will be spontaneous at
For the oxidation of glucose, ΔrG°=−3000 kJ/mol, 25% of energy is oxidised for muscle work. Therefore, in order to climb a hill of height 500 m, how many grams of glucose is required for a man of mass 100 kg? (g=10 m/s2)
If the total kinetic energy (KE) of 0.5 mole of hydrogen be the same as the total kinetic energy of 0.8 mole of Xe at 500 K, then what will be the temperature of hydrogen at that state?
The metal (M) having atomic number (Z) 26 exists in two ionic forms named Ma+ and Mb+. The magnetic moment of these ions is found to be 5.916 BM. If a > b, then the correct statement about Ma+ and Mb+ is:
Which of the following molecules has sp3d3 hybridisation and one lone pair of electrons?
Rate constant of a reaction with a virus is 3.3 × 10-4 s-1. Time required for the virus to become 75% inactivated is: (Assume that virus growth cannot take place simultaneously.)
In a nitrosyl complex, the odd electron on nitrogen of the coordinated nitrosyl is transferred to vacant (n - 1)d subshell of the central metal. Hence, the spin-only magnetic moment of the complex K[Cr(CN)4(NH3)(NO)] assuming the metal ion to be in the strong field is:


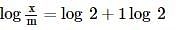

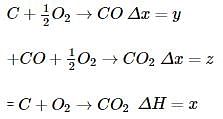
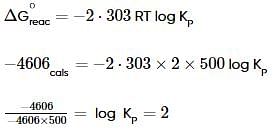


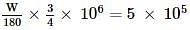
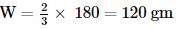
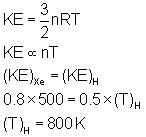
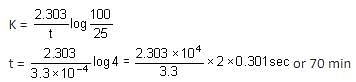
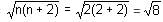 BM
BM














