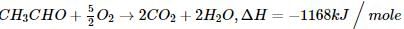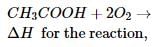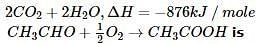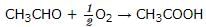BITSAT Chemistry Test - 8 - JEE MCQ
30 Questions MCQ Test - BITSAT Chemistry Test - 8
Which of the following reagents react differently with HCHO, CH₃CHO and CH₃COCH₃ ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The correct set of quantum numbers for the unpaired electron of chlorine atom is
In hydrogen atom, energy of first excited state is -3.4 eV. Then find out the K.E. of the electron in the same orbit of hydrogen atom
Which of the following has the smallest number of molecules?
Elimination of bromine from 2- bromobutane results in the formation of
The pH of blood does not appreciably change by a small addition of an acid or a base because blood
To become a carbohydrate a compound must contain at least
The standard enthalpy of formation (∆f Ho) at 298 K for CH₄(g) is -74.8 KJ mol⁻1. The additional information required to determine the average energy of C-H bond would be
If a mixture containing 3 moles of hydrogen and 1 mole of nitrogen is converted completely into ammonia, the ratio of volumes of reactants and products at the same temperature and pressure would be
Let electronegativity, ionisation energy and electron affinity be represented as EN, IP and EA respectively. Which one of the following equation is correct according to Mulliken?
Which of the following can possibly be used as analgesic without causing addiction and any modification?
Faraday's law of electrolysis states that mass deposited on electrode is proportional to
In which of the following molecules hydrogen is more acidic?
The hydrocarbon which decolourises alkaline KMnO₄ solution ,but does not give any precipitate with ammonical silver nitrate is
The pH of blood does not appreciably change by a small addition of acid or a base because blood
Under what conditions of temperature and pressure, the formation of atomic hydrogen from meolcular hydrogen will be favoured most?
The isomers, which possess same structural formula, but differ in spatial arrangement of the group around the double bond, are called
Chlorine is passed into dilute, cold KOH solution. What are the oxidation numbers of chlorine in the products formed?
What are the products formed when ammonia reacts with excess chlorine?