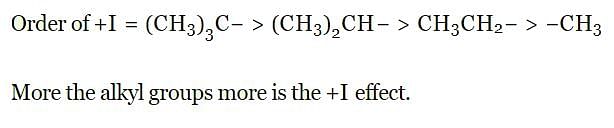BITSAT Chemistry Test - JEE MCQ
30 Questions MCQ Test BITSAT Mock Tests Series & Past Year Papers 2025 - BITSAT Chemistry Test
Which of the following reactions is used for detecting presence of carbonyl group ?
An element has 2 electrons is K-shell, 8 electrons in L-shell, 13 electrons in M-shell and one electron in N-shell. The element is
Which of the following alkyl group has the maximum + I effect?
The bromination of benzene in presence of FeBr₃ is an example of
A chemical reaction will be spontaneous if it is accompained by a decrease of
The equilibrium constant for the following reaction will be 3 A + 2 B → C
The decomposition of a substance follows first order kinetics. If its conc. is reduced to 1/8 th of its initial value, in 24 minutes, the rate constant of decomposition process is
Which of the following species has the highest ionization potential?
A complex compound of Co3⁺ with molecular formula CoClxyNH₃ gives a total of three ions per molecule when dissolved in water. How many Cl⁻ ions satisfy primary as well secondary valencies in this complex?
What would happen when a solution of potassium chromate is treated with an excess of dilute nitric acid?
Which of the following element belongs to the third row transition series?
The standard emf of the cell Zn + Cu2⁺ → Cu + Zn2 is 1.10 V at 25oC. The emf of the cell when 0.1 M Cu2⁺ and 0.1 M Zn2⁺ solutions are used will be
Which one of the following will undergo meta-substitution on monochlorination ?
Nitrogen atom has an atomic number of 7 and oxygen has an atomic number 8. The total number of electrons in a nitrate ion will be
Reaction C₂H₅I + C₅H₁₁I + 2Na → C₂H₅ - C₅H₁₁ + 2NaI is called
Amongst the compounds Mg₃N₂, NH₃ and N₂O₃, nitrogen shows an oxidation state of +3 in
|
2 videos|17 docs|85 tests
|
|
2 videos|17 docs|85 tests
|