BITSAT Mock Test - 1 - JEE MCQ
30 Questions MCQ Test BITSAT Mock Tests Series & Past Year Papers 2025 - BITSAT Mock Test - 1
A small planet is revolving around a massive star in a circular orbit of radius R with a period of revolution T. If the gravitational force between the planet and the star is proportional to R-5/2, then T will be proportional to
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A cell of constant emf is first connected to a resistance R1 and then connected to a resistance R2. If power delivered in both the cases is same, then the internal resistance of the cell is
A particle starts from rest. Its acceleration at time t = 0 is 5 ms-2, which varies with time as shown in the figure below. The maximum speed of the particle will be
Two moles of each reactant A and B are taken in a reaction flask. They react in the following manner.
A (g) + B (g)  C (g) + D (g)
C (g) + D (g)
At equilibrium, it was found that the concentration of C is triple to that of B. The equilibrium constant for the reaction is
An electron, during excitation, travels a distance of nearly 0.79 x 10-9 m in the hydrogen atom. During the de-excitation of this electron, the number of spectral lines formed is
If 75% of a first order reaction was completed in 32 minutes, then the time taken for 50% of the same reaction to be completed would be
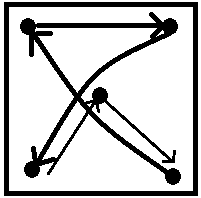
 in their positions?
in their positions?
Directions: Find the missing term in the following series.
10, 15, 25, 40, __
Directions: Find the wrong term in the following series.
49, 49, 50, 54, 60, 79, 104
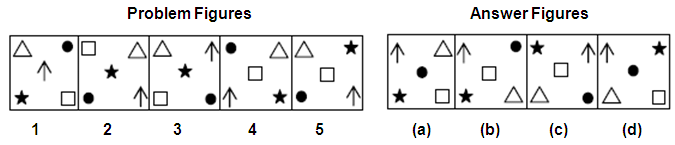
Directions: In this question, five problem figures are given followed by four answer figures (a, b, c, d). Select the figure from the answer figures which will continue the same pattern as followed in the problem figures.
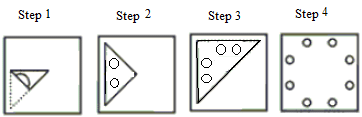
A piece of paper is folded and punched as shown below in the question figures. From the given answer figures, indicate how it will appear when opened.

The equations of normal to the curve 3x2 - y2 = 8, such that it is parallel to the line x + 3y = 4, is
The probability that a student will succeed in IIT entrance test is 0.2 and that he will succeed in Roorkee entrance test is 0.5. If the probability that he will be successful at both the places is 0.3, then the probability that he will not succeed at both the places is
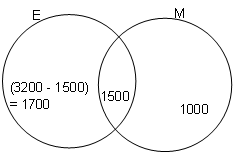
In a town with a population of 5000, the number of people who are egg eaters is 3200. If 2500 are meat eaters and 1500 eat both egg and meat, then how many of them eat neither meat nor egg?
|
2 videos|17 docs|85 tests
|
|
2 videos|17 docs|85 tests
|




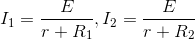








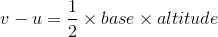
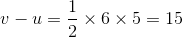

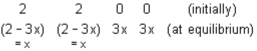
 2 - 3x = x
2 - 3x = x



 CH
CH  CH3CH2COOH + HCOOH
CH3CH2COOH + HCOOH No reaction (due to the passivity of iron)
No reaction (due to the passivity of iron) , where Ka = Equilibrium constant for weak acid
, where Ka = Equilibrium constant for weak acid = Degree of ionisation of weak acid (HA)
= Degree of ionisation of weak acid (HA) <<<< 1 or 1 -
<<<< 1 or 1 -  = 1
= 1

 = 1.34% = 0.0134
= 1.34% = 0.0134 (1.34
(1.34  10-2)2
10-2)2 10-5
10-5
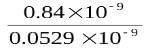 = 15.87 16
= 15.87 16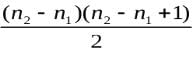 = 6
= 6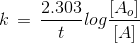
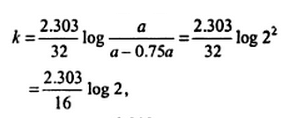
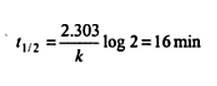

 m
m v =
v = 
 x =
x =  xA
xA v = 0.05
v = 0.05 xA
xA  m
m  0.05 =
0.05 =  ...(i)
...(i) x =
x =  xB
xB v = 0.02
v = 0.02 xB
xB  5 m
5 m  0.02 =
0.02 =  we get
we get = 2
= 2
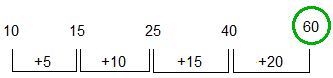
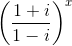 = 1, then
= 1, then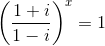
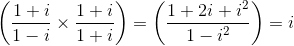
 , only when x = 4n, where n is any positive integer.
, only when x = 4n, where n is any positive integer.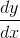 = 0
= 0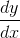 =
= 
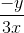
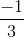
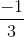 =
= 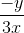
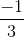 (x - x1)
(x - x1) and
and  , then the value of
, then the value of 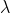 is
is



 is
is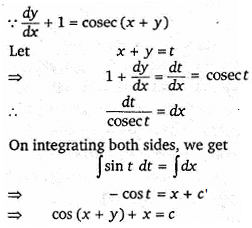

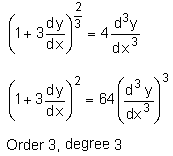
 is
is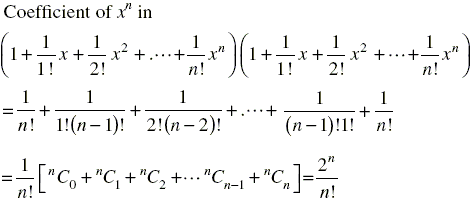
 is equal to
is equal to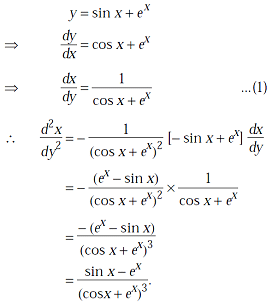
 definite sum if
definite sum if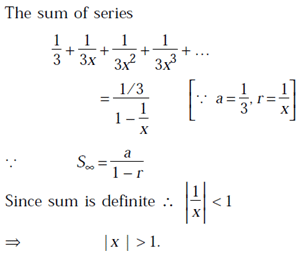
 , then
, then