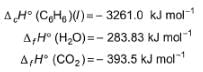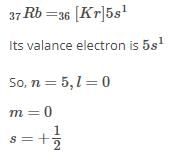Bihar STET Paper 2 Chemistry Mock Test - 6 - Bihar PGT/TGT/PRT MCQ
30 Questions MCQ Test Bihar STET Mock Test Series 2024 - Bihar STET Paper 2 Chemistry Mock Test - 6
Direction (Q. Nos. 11-14) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d)
Passage I
Solid ammonium chloride is in equilibrium with ammonia and hydrogen chloride gases
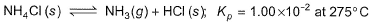
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
Q. Partial pressure of NH3(g) or HCI (g) at equilibrium is
0.980 g of solid NH4CI is taken in a closed vessel of 1 L capacity and heated to 275° C.
The correct order of thermal stability of the hydrides of group 16 elements is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
As you move from left to right across the periodic table:
Which among the following is an example of pseudo first order reaction?
For the reaction system: 2NO(g) + O2(g) → 2NO2(g) volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with repect to NO, the rate of reaction will –
[AIEEE-2003]
Thermal decomposition of a compound is of first order. If 50% of a sample of a compound is decomposed in 120 min, the time taken for 90% completion is
[Ti (H2O)6]3+ absorbs green and yellow region part of visible light. Then the transmitted colour of the compound is
In the electrolysis of aqueous sodium chloride solution, two types of reactions can take place at anode :
I. 2Cl- (aq) → Cl2(g) +2e-
II. 2H2O(l)g → O2 (g) + 4H+(aq) + 4e-
Select the correct statement(s) about these.
Among the following the element with highest first ionization energy is:
Which of the following equation depicts reducing nature of H2O2?
Noble gases are inert and do not form compounds like other elements because of their:
One or More than One Options Correct Type
This section contains 3 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Following solutions have been provided at temperature T K.
I. 1M aqueous glucose solution.
II. 1M aqueous sodium chloride solution.
III. 1M aqueous ammonium phosphate solution.
IV. 1M benzoic acid in benzene.
Select correct statements for the above solutions.
Number of atoms is 560 g of Fe (atomic mass = 56 g mol-1)
How many atoms of hydrogen are in 67.2 L of H2 at STP?
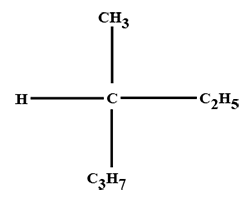
The minimum number of C atoms required for a hydrocarbon to exhibit optical isomerism:
A mixture of ethyl alcohol and propyl alcohol has a vapour pressure of 290 mm at 300 K. The vapour pressure of propyl alcohol is 200 mm. If the mole fraction of ethyl alcohol is 0.6, its vapour pressure (in mm) at the same temperature will be -
[AIEEE 2007]
The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is
[JEE Main 2013]
The complex [Co(NH3)6[Cr(C2O4)3] and [Cr(NH3)6[Co(C2O4)3] exhibit
Direction:
This section contains 5 questions. When worked out will result in an integer from 0 to 9 {both inclusive).
Q.
Relative decrease in vapour pressure of an aqueous NaCI solution is 0.167. Thus, number of moles of NaCI present in 180 g of H20 is ... .
For an aqueous solution, freezing point is _0.186ºC . Boiling point of the same solution is
(Kƒ = 1.86º K mol-1 kg) and Kb = 0.512º K mol-1 kg)
[AIEEE-2002]
Which one of the following aqueous solutions will exhibit highest boiling point ?
[AIEEE-2004]
In which of the following compounds the carbon marked with asterisk is expected to have greatest positive charge?
What is/are the advantages of Play-way method of teaching in a classroom?
I. Learning becomes natural, joyful and energizing experience
II. It provides scope to the children to fulfil their physical, emotional and cognitive needs
III. It helps to build healthy student-teacher and student-student relationships
To make assessment 'a useful and interesting process', one should be careful about:
In the villages of Bihar, many farmers do bee-keeping and collect honey to earn extra money. The best time to start bee-keeping is: