Biotechnology - 2014 Past Year Paper - IIT JAM MCQ
30 Questions MCQ Test IIT JAM Past Year Papers and Model Test Paper (All Branches) - Biotechnology - 2014 Past Year Paper
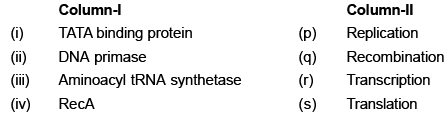
Match the proteins listed in Column- I with their major cellular function in Column- II:
Amongst the following, the elongated, fibrous protein is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The mutation likely to cause the least perturbation in the tertiary structure of a protein is
Match the techniques in Column- I with their primary applications in Column- II:
Amongst the following statements about biological membranes, the INCORRECT one is that they
The introduction of a new fish species into a lake resulting in the extinction of several native fish species from the lake is an example of
The taxonomic hierarchy in descending order of size is
If the recessive disease phenylketonuria (PKU) occurs in a genetically constant population with a frequency of 1 in 10000, the frequency of the carrier genotype is
Amplification of a DNA fragment by PCR yields only one faint band of the expected size on an agarose gel. For such a sample, the best way to increase the yield of the PCR product is to
The cellular organelle which function(s) as a store for Ca2+ ion is
If the N- terminal 21 amino acids were missing from a mitochondrial protein, its cellular location after synthesis would be
Packaged biomaterials are dispatched to intracellular and extracellular locations from the
The binding of a hormone to its receptor activates adenylyl cyclase through a stimulatory G protein. If, due to a mutation, the G- protein binds but does NOT hydrolyze GTP, the consequence will be
A toxin which causes accumulation of twice the normal amount of DNA in a dividing mammalian cell, most likely blocks the cell cycle
Bt toxin, produced by Bacillus thuringiensis, does NOT kill the bacteria itself because the toxin is
The inactivation of an mRNA due to its binding to a complementary RNA molecule is called
Given are the sequences of one strand of double-stranded DNA. The one with the highest melting point (Tm) is
The standard pregnancy kit, used to detect Human Chorionic Gonadotrophin (HCG) in urine, is based on
The preferred system of large- scale production of influenze virus for vaccination is
A monoclonal antibody produced against a small peptide derived from protein X, is unable to bind X in an ELISA. This is because
The lac repressor is produced from a stretch of DNA called the
The correct ascending order of melting points of oleic acid (O), linoleic acid (L), palmitic acid (P) and stearic acid (L), palmitic acid (P) and stearic acid (S) is
A peptide Glu- His- Trp- Ser- Gly- Leu- Arg- Pro- Gly, having an isoelectric point of 7.8, is placed in an electric field at pH 3.0. It will migrate towards
X- ray diffraction of wool shows repeated structural units speed at 5.2 Å, which is changed to 7.0 Å on steaming. This is due to the conversion of secondary structure from
At Et = 20 nm and substrate concentration = 40 mM, the reaction velocity V0 of an enzyme is 9.6 mM s-1. Assuming kcat to be 600 s-1, the KM will be
Which of the following statements is NOT true for an enzyme catalyzed reaction?
Which of the following is NOT an allosteric regulatory enzyme in glycolysis?
Match the enzymes of TCA cycle in Group- I with that of their products listed in Group- II
|
29 docs|48 tests
|
|
29 docs|48 tests
|

















