CPU - Redox Reaction - Retro JEE Main - Class 11 MCQ
9 Questions MCQ Test - CPU - Redox Reaction - Retro JEE Main
Retro JEE Main (Compilation of Last 13 Years Questions)
IIT JEE (Main & Advanced) past year questions with solutions & answer key
Retro IIT JEE (Main and Advanced) have questions from previous/past (10 to 13 years) papers
of IIT JEE (Main and Advanced) with solutions,
The following set of questions contain previous/past (10 to 13 years) questions of IIT JEE
(Main and Advanced) of Chapter "Redox Reaction" with solutions
Q. In which of the following reactions H2O2 acts as a reducing agent?
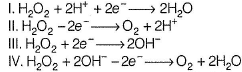
of IIT JEE (Main and Advanced) with solutions,
(Main and Advanced) of Chapter "Redox Reaction" with solutions
Consider the following reaction,
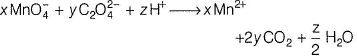
The values of x, y and z in the reaction are, respectively
(AIEEE 2013)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following chemical reactions depicts the oxidising behaviour of H2SO4?
(AIEEE 2006)
The oxidation state of Cr in [Cr(NH3)4CI2]+ is
[AIEEE 2005]
The oxidation state of chromium in the final product formed by the reaction between Kl and acidified potassium dichromate solution is
(AIEEE 2005)
Oxidation number of Cl in CaOCI2 (bleaching powder) is
(AIEEE 2002)
is a good oxidising agent in different medium changing to
Changes in oxidation number respectively, are
(AIEEE 2002)
Which of the following is a redox reaction ?
(AIEEE 2002)
Which of the following reaction is possible at anode ?
(AIEEE 2002)

















