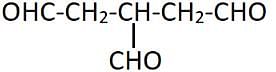Carbonyl Group MCQ - 1 (Advanced) - JEE MCQ
25 Questions MCQ Test - Carbonyl Group MCQ - 1 (Advanced)
Nucleophilic substitution at acyl carbon of a carboxylic acid derivative generally proceeds by.
The correct order of decreasing reactivity of the given compound towards hydrolysis under identical condition is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following proposed reactions would take place most quickly under mild conditions ?
In the given reaction sequene :
C6H5_CN A
B
C, (C) is
List the following esters in order of decreasing reactivity in the second step of a nucleophilic acyl substitution reaction.
(I) (II)
(III) (IV)
Select the correct answer from the codes given below:
Which one of the following is least reactive for hydrolysis reaction ?
Which one of the following esters is most reactive for saponification ?
Consider the following statements for hydrolysis reaction :
(I) is more reactive than C6H5COOC2H5
(II) is more reactive than
(III) is more reactive than
Of these the correct statements are
Ease of esterification of following acids with CH3OH
(I) HCOOH (II) CH3 COOH (III) CH3 – CH2 – COOH (IV) CH3 – – COOH, is
In the given reaction
C2H5–O––O–C2H5+HO–CH 2–CH2–OH
[X], [X] is
Ease of esterification of following alcohol with HCOOH is
(I) CH3–CH2OH (II) (CH3)2CH–OH (III) (CH3)3C–OH