Carbonyl Group MCQ - 1 (Competition Level) - Class 12 MCQ
30 Questions MCQ Test - Carbonyl Group MCQ - 1 (Competition Level)
Which of the following acids has the smallest dissociation constant ?
Which compound should have zero dipole moment ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When picric acid is treated with aqueous sodium bicarbonate (NaH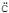 O3) a gas is liberated with effervessence. The gas evolved would be :
O3) a gas is liberated with effervessence. The gas evolved would be :
In the following ester there are three carbon oxygen bonds denoted by x, y and z
Their lengths are in order
(C – O) bond lengths designated by A,B,C are in order :
Cannizzaro’s reaction is not given by _____________.
The acid D obtained through the following sequence of reactions is -
C2H5Br A
B
C D
Identify X in the reaction sequence
X
HOOC–CH2 – CH2–COOH
The final product of the following reaction sequence is
X
In the above reaction sequence X & Y are.
A racemic mixture of carboxylic acid having one chiral centre is treated with enantiomerically pure amine. the products formed are :
The product of the following reaction is
CH3–COOH
P
Addition of water to alkynes occurs in acidic medium and in the presence of Hg2+ ions as a catalyst. Which of the following products will be formed on addition of water to but-1-yne under these conditions.
For the following conversion the correct sequence of reacgent is
In the given reaction
CH3COOH [x], [x] will be
In the reaction sequence CH3–CH2–COOH x
y
The correct sequence of reagent for the following conversion.
is
+
x + y the organic products x and y are :
For the following acids the rate of decarboxylation on heating would be :
I. II.
III. NO2–CH2–COOH IV. HOOC–CH2–COOH
End product of the following reaction is
CH3CH2COOH
In the anion HCOO the two carbon-oxygen bonds are found to be of equal length. What is the reason for it ?
When CH2 = CHCOOH is reduced with LiAlH4, the compound obtained is :

















