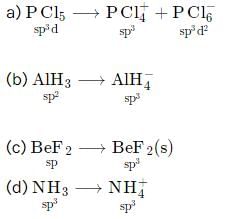Chemical Bonding (Chapter Test - Non-Medical) - Class 12 MCQ
20 Questions MCQ Test - Chemical Bonding (Chapter Test - Non-Medical)
Coordinate covalent bond is absent in
Among the following species, the least angle around the central atom is in
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following compounds have the same number of lone pairs with their central atom ?
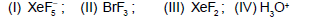
Give the correct order of initials T or F for following statements. Use T, if statement is true and F, if it is false
(I) The order of repulsion between different pair of electrons, is lp - lp > lp - bp > bp - bp
(II) In general, as the number of lone pair of electrons on central atom increases, value of bond angle increases
(III) The number of lone pair on O in H2O is 2 while on N in NH3 is 1
(IV) The structures of xenon fluorides and xenon oxyfluorides could not be explained on the basis of VSEPR theory
N2 and O2 are converted into monoanions and
respectively. Which of the following statements is wrong?
Which of the following shows the incorrect order of decreasing covalent character ?
The hybridization of atomic orbitals of nitrogen inare
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2 D). This is beacuse
In which of the process, the bond order increases and magnetic behaviour changes ?
Which are true statements among the following ?
I. PH5 and BiCl5 do not exist
II. pπ–dπ bond is present in SO2
III. Electrons travel with speed of light
IV. SeF4 and CH4 has same shape
V. I3+ has bent shape
Hybridization on carbon in short-lived species is
The correct order of dipole moment is
Which of the following is not linear ?
Which of the following statements is correct for CsBr3 ?
Which has triangular planar shape ?
In which of the following process hybridization is not affected?