Chemical Engineering- CH 2012 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemical Engineering- CH 2012 GATE Paper (Practice Test)
Q. 1 – Q. 5 carry one mark each.
Q. Which one of the following options is the closest in meaning to the word given below?
Mitigate
Q. Which one of the following options is the closest in meaning to the word given below?
Mitigate
C hoose the most appropriate alternative from the options given below to complete the following
sentence:
Despite several ––––––––– the mission succeeded in its attempt to resolve the conflict.
sentence:
Despite several ––––––––– the mission succeeded in its attempt to resolve the conflict.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The cost function for a product in a firm is given by 5q2, where q is the amount of production. The firm can sell the product at a market price of 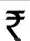 50 per unit. The number of units to be produced by the firm such that the profit is maximized is
50 per unit. The number of units to be produced by the firm such that the profit is maximized is
C hoose the most appropriate alternative from the options given below to complete the following
sentence:
Suresh’s dog is the one ––––––––– was hurt in the stampede
C hoose the grammatically INCORRECT sentence:
Q. 6 - Q. 10 carry two marks each.
Q.
A n automobile plant contracted to buy shock absorbers from two suppliers X and Y. X supplies
60% and Y supplies 40% of the shock absorbers. All shock absorbers are subjected to a quality test.
The ones that pass the quality test are considered reliable. Of X’s shock absorbers, 96% are reliable.
Of Y’s shock absorbers, 72% are reliable.
The probability that a randomly chosen shock absorber, which is found to be reliable, is made by
Y is
A political party orders an arch for the entrance to the ground in which the annual convention is
being held. The profile of the arch follows the equation y = 2x – 0.1x2 where y is the height of the
arch in meters. The maximum possible height of the arch is
W anted Temporary, Part-time persons for the post of Field Interviewer to conduct personal
interviews to collect and collate economic data. Requirements: High School-pass, must be
available for Day, Evening and Saturday work. Transportation paid, expenses reimbursed.
Which one of the following is the best inference from the above advertisement?
G iven the sequence of terms, AD CG FK JP, the next term is
W hich of the following assertions are CORRECT?
P: Adding 7 to each entry in a list adds 7 to the mean of the list
Q: Adding 7 to each entry in a list adds 7 to the standard deviation of the list
R: Doubling each entry in a list doubles the mean of the list
S: Doubling each entry in a list leaves the standard deviation of the list unchanged
Q. 11 – Q. 35 carry one mark each.
Q.
Consider the following set of linear algebraic equations
The system has
If a and b are arbitrary constants, then the solution to the ordinary differential equation
is
F or the function the Taylor series approximation for
is.
A box containing 10 identical compartments has 6 red balls and 2 blue balls. If each compartment
can hold only one ball, then the number of different possible arrangements are
C onsider the following (2 x 2) matrix
Which one of the following vectors is NOT a valid eigenvector of the above matrix ?
In a throttling process, the pressure of an ideal gas reduces by 50 %. If CP and CV are the heat
capacities at constant pressure and constant volume, respectively the specific
volume will change by a factor of
I f the temperature of saturated water is increased infinitesimally at constant entropy, the resulting
state of water will be
In a parallel flow heat exchanger operating under steady state, hot liquid enters at a temperature
Th,in and leaves at a temperature Th,out . Cold liquid enters at a temperature Tc,in and leaves at a
temperature Tc,out . Neglect any heat loss from the heat exchanger to the surrounding. If
Th,in >> Tc,in , then for a given time interval, which ONE of the following statements is true?
F or an exothermic reversible reaction, which one of the following correctly describes the
dependence of the equilibrium constant ( K ) with temperature (T ) and pressure ( P ) ?
W ater is flowing under laminar conditions in a pipe of length L . If the diameter of the pipe is
doubled, for a constant volumetric flow rate, the pressure drop across the pipe
The local velocity of a fluid along a streamline can be measured by
F or uniform laminar flow (in the x -direction) past a flat plate at high Reynolds number, the local
boundary layer thickness (δ ) varies with the distance along the plate ( x ) as
I n a mixing tank operating at very high Reynolds number (> 104 ), if the diameter of the impeller is
doubled (other conditions remaining constant), the power required increases by a factor of
F or heat transfer across a solid-fluid interface, which one of the following statements is NOT true
when the Biot number is very small compared to 1?
A solid sphere with an initial temperature Ti is immersed in a large thermal reservoir of
temperature To . The sphere reaches a steady temperature after a certain time t1 . If the radius of the
sphere is doubled, the time required to reach steady-state will be
I f the Nusselt number ( Nu ) for heat transfer in a pipe varies with Reynolds number (Re ) as
then for constant average velocity in the pipe, the heat transfer coefficient varies with
the pipe diameter D as
I n the McCabe-Thiele diagram, if the x -coordinate of the point of intersection of the q -line and
the vapor-liquid equilibrium curve is greater than the x -coordinate of the feed point, then the
quality of the feed is
F or which of the following combinations, does the absorption operation become gas-film
controlled?
P. The solubility of gas in the liquid is very high
Q. The solubility of gas in the liquid is very low
R. The liquid-side mass transfer coefficient is much higher than the gas-side mass transfer
coefficient
S. The liquid-side mass transfer coefficient is much lower than the gas-side mass transfer
coefficient
The half-life of an nth order reaction in a batch reactor depends on
C onsider the reaction scheme shown below
Both the reactions are first-order. The activation energies for k1 and k2 are 80 and 20 kJ/mol,
respectively. To maximize the yield of B, it is preferable to use

















