Chemical Engineering- CH 2013 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemical Engineering- CH 2013 GATE Paper (Practice Test)
Q. No. 1 – 5 Carry One Mark Each
Q.
If 3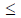 X
X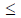 5 and 8
5 and 8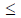 Y
Y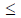 11 then which of the following options is TRUE?
11 then which of the following options is TRUE?
The Headmaster to speak to you.
Which of the following options is incorrect to complete the above sentence?
Which of the following options is incorrect to complete the above sentence?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Mahatama Gandhi was known for his humility as
"All engineering students" "should learn mechanics", "mathematics and" "how to do computation."
I II III IV
Which of the above underlined parts of the sentence is not appropriate?
Select the pair that best expresses a relationship similar to that expressed in the
pair:
water: pipe::
Q. No. 6 – 10 Carry Two Marks Each
Q.
Velocity of an object fired directly in upward direction is given by V = 80 − 32 t,
where t (time) is in seconds. When will the velocity be between 32 m/sec and 64
m/sec?
In a factory, two machines M1 and M2 manufacture 60% and 40% of the autocomponents respectively. Out of the total production, 2% of M1 and 3% of M2 are found to be defective. If a randomly drawn autocomponent from the combined lot is found defective, what is the probability that it was manufactured
by M2?
Following table gives data on tourists from different countries visiting India in the
year 2011.
Which two countries contributed to the one third of the total number of tourists
who visited India in 2011?
If |−2X + 9| = 3 then the possible value of |−X| − X2 would be:
All professors are researchers
Some scientists are professors
Which of the given conclusions is logically valid and is inferred from the above
arguments:
Q. No. 11 – 25 Carry One Mark Each
Q.
The number of emails received on six consecutive days is 11, 9, 18, 18, 4 and 15,
respectively.
What are the median and the mode for these data?
For two rolls of a fair die, the probability of getting a 4 in the first roll and a
number less than 4 in the second roll, up to 3 digits after the decimal point, is _______
Imp : you should answer only the numeric value
Which of the following statements are TRUE?
P. The eigen values of a symmetric matrix are real
Q. The value of the determinant of an orthogonal matrix can only be +1
R. The transpose of a square matrix A has the same eigen values as those of A
S. The inverse of an ‘n × n’ matrix exists if and only if the rank is less than ‘n’
Evaluate
(Note: C is a constant of integration)
A gaseous system contains H2, I2, and HI, which participate in the gas-phase
reaction
At a state of reaction equilibrium, the number of thermodynamic degrees of
freedom is
Imp : you should answer only the numeric value
The thermodynamic state of a closed system containing a pure fluid changes from
(T1, p1) to (T2, p2), where T and p denote the temperature and pressure,
respectively. Let Q denote the heat absorbed (> 0 if absorbed by the system) and
W the work done (> 0 if done by the system). Neglect changes in kinetic and
potential energies. Which one of the following is CORRECT?
An equation of state is explicit in pressure p and cubic in the specific volume v. At
the critical point ‘c’, the isotherm passing through ‘c’ satisfies
The units of the isothermal compressibility are
An open tank contains two immiscible liquids of densities (800 kg/m3 and 1000 kg/m3) as shown in the figure. If g = 10 m/s2, under static conditions, the gauge pressure at the bottom of the tank in Pa is _____________
Important : you should answer only the numeric value
The apparent viscosity of a fluid is given by is the velocity gradient. The fluid is
The mass balance for a fluid with density (ρ) and velocity vector is
An incompressible Newtonian fluid, filled in an annular gap between two concentric cylinders of radii R1 and R2 as shown in the figure, is flowing under steady state conditions. The outer cylinder is rotating with an angular velocity of ? while the inner cylinder is stationary. Given that ( R1-R2) ? R1 the profile of the θ -component of the velocity Vθ can be approximated by,
For a Newtonian fluid flowing in a circular pipe under steady state conditions in
fully developed laminar flow, the Fanning friction factor is
In the Tyler standard screen scale series, when the mesh number increases from
3 mesh to 10 mesh, then
Taking the acceleration due to gravity to be 10 m/s2, the separation factor of a cyclone 0.5 m in diameter and having a tangential velocity of 20 m/s near the wall is_____
Important : you should answer only the numeric value
The effectiveness of a heat exchanger in the ε − NTU method is defined as
In a pool boiling experiment, the following phenomena were observed.
P. Natural convection
Q. Film boiling
R. Transition boiling
S. Nucleate boiling
What was the CORRECT sequence of their occurrence?
A hole of area 1 cm2 is opened on the surface of a large spherical cavity whose inside temperature is maintained at 727 °C. The value of Stefan-Boltzmann constant is 5.67×10-8 W/m2-K4. Assuming black body radiation, the rate at which the energy is emitted (in W) by the cavity through the hole, up to 3 digits after the decimal point, is __________
Important : you should answer only the numeric value
The packing of an existing absorption tower is replaced with a new type of packing. The height of the packing and the inlet conditions are maintained the same as before. Tests reveal that the number of transfer units is lower than before. This indicates that the tower with the new packing, when compared to
that with the old packing, will
A wet solid is dried over a long period of time by unsaturated air of nonzero constant relative humidity. The moisture content eventually attained by the solid is termed as the

















