Chemical Engineering- CH 2017 GATE Paper MCQ Quiz - GATE MCQ
30 Questions MCQ Test - Chemical Engineering- CH 2017 GATE Paper MCQ Quiz
In a venturi meter, ∆P1 and ∆P2 are the pressure drops corresponding to volumetric flowrates Q1 and Q2. If 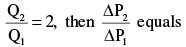
The volumetric properties of two gases M and N are described by the generalized compressibility chart which expresses the compressibility factor (Z) as a function of reduced pressure and reduced temperature only. The operating pressure (P) and temperature (T) of two gases M and N along with their critical properties (PC , TC) are given in the table below.

ZM and ZN are the compressibility factor of the gases M and N under the given operating conditions respectively.
The relation between ZM and ZN is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Match the polymerization process in Group-1 with the polymers in Group-2.

Choose the correct set of combinations.
An LVDT (Linear Variable Differential Transformer) is a transducer used for converting
Match the variables in Group-1 with the instruments in Group-2.
Choose the correct set of combinations.
The thickness of laminar boundary layer over a flat plate varies along the distance from the leading edge of the plate. As the distance increases, the boundary layer thickness
The marks obtained by a set of students are 38, 84, 45,70, 75, 60, 48. The mean and median marks, respectively, are
In a heat exchanger, the inner diameter of a tube is 25 mm and its outer diameter is 30 mm. The overall heat transfer coefficient based on the inner area is 360 W/m2.°C. Then, the overall heat transfer coefficient based on the outer area, rounded to the nearest integer, is ___ W/m2.°C.
The cost of a new pump (including installation) is 24,000 Rupees. The pump has useful life of 10 years. Its salvage value is 4000 Rupees. Assuming straight line depreciation, the book value of the pump at the end of 4th year, rounded to the nearest integer, is ___ Rupees.
A gas bubble (gas density ρg = 2 kg / m3; bubble diameter D = 10−4 m) is rising vertically through water (density ρ = 1000 kg / m3 ; viscosity µ = 0.001 Pa.s). Force balance on the bubble leads to the following equation,
Where ν is the velocity of the bubble at any given time t. Assume that the volume of the rising bubble does not change. The value of g = 9.81m / s2. The terminal rising velocity of the bubble (in cm/s), rounded to 2 decimal places, is ___ cm/s.
Water is heated at atmospheric pressure from 40°C to 80°C using two different processes. In process I, the heating is done by a source at 80°C. In process II, the water is first heated from 40°C to 60°C by a source at 60°C, and then from 60°C to 80°C by another source at 80°C. Identify the correct statement.
The DCDA (Double Contact Double Absorption) process is used the manufacture of
The real part of 6eiπ/3 is ___.
Consider a first order catalytic reaction in a porous catalyst pellet.
Given R - characteristic length of the pellet: De - effective diffusivity; kC - mass transfer coefficient: k1 - rate constant based on volume of the catalyst pellet; Cs - concentration of reactant on the pellet surface.
The expression for Thiele modulus is
Which of the following conditions are valid at the plait point?
P) Density difference between the extract and raffinate phases is zero
Q) Interfacial tension between the extract and raffinate phases is zero
R) Composition difference between the extract and raffinate phases is zero
The purpose of the mathanation reaction used in ammonia plants is to
Which of the following is the correct sequence of equipment for size reduction of solids?
Let i and j be the unit vectors in the x and y directions, respectively. For the function F(x, y) = x3 + y3 the gradient of the function i.e.. ∇F is given by
Consider steady state mass transfer of a solute A from a gas phase to a liquid phase. The gas phase bulk and interface mole fractions are yA,G and yA,i respectively. The liquid phase bulk and interface mole fractions are xA , L and xA ,i , respectively. The ratio close to zero.
The implies that mass transfer resistance is
For a solid-catalyzed gas phase reversible reaction, which of the following statements is ALWAYS TRUE?
The one-dimensional unsteady heat conduction equation is
Where, T – temperature, t – time, r – radial position, k – thermal conductivity, ρ- density, and
Cp - specific heat.
For the cylindrical coordinate system, the value of n in the above equation is
The composition of vapour entering a tray in a distillation column is 0.47. The average composition of the vapour leaving the tray is 0.53. The equilibrium composition of the vapour corresponding to the liquid leaving this tray is 0.52. All the compositions are expressed in mole fraction of the more volatile component.
The Murphree efficiency based on the vapour phase, rounded to the nearest integer, is ___ %
The number of positive roots of the function f (x) shown below in the range 0 < x < 6 is ___________.
The following reaction rate curve is shown for a reaction A → P. Here, (−rA) and xA represent reaction rate and conversion, respectively. The feed is pure A and 90% conversion is desired
Q. Which amongst the following reactor configurations gives the lowest total volume of the reactor (s)?
The total cost (CT) of an equipment in terms of the operation variables x and y is
The optimal value of CT, rounded to 1 decimal place, is ____.
The vapour pressure of a pure substance at a temperature T is 30bar. The actual and ideal gas values of g / RT for the saturated vapour at this temperature T and 30 bar are 7.0 and 7.7 respectively. Here, g is the molar Gibbs free energy and R is the universal gas constant. The fugacity of the saturated liquid at these conditions, rounded to 1 decimal place, is ___ bar.
The Sherwood number (ShL) correlation for laminar flow over a flat plate of length L is given by
Where ReL and Sc represent Reynolds number and Schmidt number, respectively. This correlation, expressed in the form of Chilton-Colburn jD factor, is
For the initial value problem the value of x at
In a counter current stripping operation using pure steam the mole ratio of a solute in the liquid stream is reduced from 0.25 to 0.05. The liquid feed flowrate, on a solute-free basis, is 3mole/s. The equilibrium line for the system is given in the figure below.
The MINIMUM flowrate of pure steam for this process, rounded to 1 decimal place, is ___ mol/s.

















