Chemical Equation MCQ - 2 (Advanced) - JEE MCQ
20 Questions MCQ Test - Chemical Equation MCQ - 2 (Advanced)
Solid ammonium carbamate, NH4 CO2NH2 (s) dissociates into ammonia and carbon dioxide when it evaporates as shown by
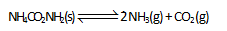
'the total pressure of the gases in equilibrium with the solid is 0.116 atm. If 0.1 atm of CO2 is introduced after equilibrium is reached then :
For the equilibrium,
PCI3 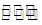 PCI3 (g) + CI2 (g)
PCI3 (g) + CI2 (g)
Which of the following sketch may represent above equilibrium. Assume equilibrium can be achieved from either side and by taking any one or more components initially. (Given Kcfor the reaction < 2)
138 gm of N2O4 (g) is placed in 8.2L container at 300 K. The equilibrium vapour density of mixture was found to be 30.67. Then (R = 0.082 L atm mol-1K–1)
Solid ammonium carbamate dissociate to give ammonia and carbon dioxide as follows NH2COONH4(s) 2NH3(g) + CO2(g)
which of the following graph incorrectly represents the equilibrium.
2CaSO4 (s) ˆˆ ˆˆ ˆˆˆˆ 2Ca0(s) + 2SO2(g) + O2(g), ΔH > 0
Above equilibrium is established by taking sufficient amount of CaSO4(s) in a closed container at 1600 K. Then which of the following may be correct option(Assume thatsolid CaSO4 is present in the container in each case).
CuSO4 5H2O(s) CuSO4(s) + 5H2O(g)
Kp = 10-10 (atm). 10–2 moles of CuSO4 5H2O(s) is taken in a 2.5L container at 27°C then at equilibrium [Take : R = 1/12 litre atm mol–1 K–1]
A closed jar having waters vapours in equilibrium with liquid suddenly all the vapours of the jar is transferred to another,identical jar and is subjected to compression. Assume initial temperature to be the same and negligible volume occupied by the liquid water. Select the observation in the record jar
For a reversible reaction ad + bB cC + dD) ; the variation of K with temperature is
given by
If two gases ABA and 62C are mixed the following equilibria are readily Established
AB2 (9) + B2C(g) → AB3 (g) + BC (g)
BC(g) + B2C(g) → B3C2 (g)
It the reaction is started only with AB2 with B2C, then which of the following is necessarily true at equilibrium:
The following reaction attains equilibrium at high temperature
.N2(g) + 2H2 OP(g) + heat 2NO(g) + 2H2 (g)
The yield of NO is affected by
If a systemat equilibrium is subjected to a change of any one of the factors such as concentration, pressure temperature, the system adjusts itself in such a way as to (Nulify) the effect of that change.
Change of pressure : If a system in equilibrium consists of gases, then the concentrations of all the components can be altered by changing the pressure. When the pressure on the system is increased, the volume decreases proportionately. The total number of moles per unit volume will now be more and the equilibirum will shift in the direction in which there is decrease in number of moles ice., towards the direction in which there is decrease in volume.
Effect of pressure on melting point : There are two types of solids : (a) Solids whose volume decreases on melting, egg., ice, diamond, carborundum, magnesium nitride and quartz Solid (higher volume) Liquid (lower volume) Them process of melting is facilitated at high pressure, thus melting point is lowered. .(b) Solids whose volume increase on melting, e.g., Fe, Cu, Ag, Au, etc.
Solid (lower volume) Liquid (higher volume) In this case the process of melting become difficult at high pressure; thus melting point becomes
high.
(c) Solubility of substances : When solid substance are dissolved in water, either heat is evolved (exothermic) or heat is absorbed (endothermic).
KCI + aq KCl(aq) – heat In such cases, solubility increase with increase in temperature. Consider the case of KOH; when this is dissolved, heat is evolved.
KOH + aq KOH(aq) + heat In such cases, solubility decrease with increase in temperature. (d) Solubility of gases in liquids : When a gas dissolves in liquid, there is decrease in volume. Thus, increase of pressure will favour the dissolution of gas in liquid.
Effect of temperature : Le-Chatelier's principle predicts a system at equilibrium will tend to shift in the endothermic direction when temperature is raised, for then energy is absorbed as heat and the rise in temperature is opposed. Conversely, an equilibrium will shift in the exothermic direction if the temperature is lowered, for then that energy is released and the reduction in temperature is opposed. Van't Hoff equation shows the dependence of equilibrium constant K on temperature as:
Q.
A gas 'X when dissolved in water heat is evolved. Then solubluty of 'X' will Increase
If a systemat equilibrium is subjected to a change of any one of the factors such as concentration, pressure temperature, the system adjusts itself in such a way as to (Nulify) the effect of that change.
Change of pressure : If a system in equilibrium consists of gases, then the concentrations of all the components can be altered by changing the pressure. When the pressure on the system is increased, the volume decreases proportionately. The total number of moles per unit volume will now be more and the equilibirum will shift in the direction in which there is decrease in number of moles ice., towards the direction in which there is decrease in volume.
Effect of pressure on melting point : There are two types of solids : (a) Solids whose volume decreases on melting, egg., ice, diamond, carborundum, magnesium nitride and quartz Solid (higher volume) Liquid (lower volume) Them process of melting is facilitated at high pressure, thus melting point is lowered. .(b) Solids whose volume increase on melting, e.g., Fe, Cu, Ag, Au, etc.
Solid (lower volume) Liquid (higher volume) In this case the process of melting become difficult at high pressure; thus melting point becomes
high.
(c) Solubility of substances : When solid substance are dissolved in water, either heat is evolved (exothermic) or heat is absorbed (endothermic).
KCI + aq KCl(aq) – heat In such cases, solubility increase with increase in temperature. Consider the case of KOH; when this is dissolved, heat is evolved.
KOH + aq KOH(aq) + heat In such cases, solubility decrease with increase in temperature. (d) Solubility of gases in liquids : When a gas dissolves in liquid, there is decrease in volume. Thus, increase of pressure will favour the dissolution of gas in liquid.
Effect of temperature : Le-Chatelier's principle predicts a system at equilibrium will tend to shift in the endothermic direction when temperature is raised, for then energy is absorbed as heat and the rise in temperature is opposed. Conversely, an equilibrium will shift in the exothermic direction if the temperature is lowered, for then that energy is released and the reduction in temperature is opposed. Van't Hoff equation shows the dependence of equilibrium constant K on temperature as:
Q.
Above equilibrium is favoured at :
If a systemat equilibrium is subjected to a change of any one of the factors such as concentration, pressure temperature, the system adjusts itself in such a way as to (Nulify) the effect of that change.
Change of pressure : If a system in equilibrium consists of gases, then the concentrations of all the components can be altered by changing the pressure. When the pressure on the system is increased, the volume decreases proportionately. The total number of moles per unit volume will now be more and the equilibirum will shift in the direction in which there is decrease in number of moles ice., towards the direction in which there is decrease in volume.
Effect of pressure on melting point : There are two types of solids : (a) Solids whose volume decreases on melting, egg., ice, diamond, carborundum, magnesium nitride and quartz Solid (higher volume) Liquid (lower volume) Them process of melting is facilitated at high pressure, thus melting point is lowered. .(b) Solids whose volume increase on melting, e.g., Fe, Cu, Ag, Au, etc.
Solid (lower volume) Liquid (higher volume) In this case the process of melting become difficult at high pressure; thus melting point becomes
high.
(c) Solubility of substances : When solid substance are dissolved in water, either heat is evolved (exothermic) or heat is absorbed (endothermic).
KCI + aq KCl(aq) – heat In such cases, solubility increase with increase in temperature. Consider the case of KOH; when this is dissolved, heat is evolved.
KOH + aq KOH(aq) + heat In such cases, solubility decrease with increase in temperature. (d) Solubility of gases in liquids : When a gas dissolves in liquid, there is decrease in volume. Thus, increase of pressure will favour the dissolution of gas in liquid.
Effect of temperature : Le-Chatelier's principle predicts a system at equilibrium will tend to shift in the endothermic direction when temperature is raised, for then energy is absorbed as heat and the rise in temperature is opposed. Conversely, an equilibrium will shift in the exothermic direction if the temperature is lowered, for then that energy is released and the reduction in temperature is opposed. Van't Hoff equation shows the dependence of equilibrium constant K on temperature as:
Q.
For the reaction,
pressure is increased by reducing the volume of the container then
If a systemat equilibrium is subjected to a change of any one of the factors such as concentration, pressure temperature, the system adjusts itself in such a way as to (Nulify) the effect of that change.
Change of pressure : If a system in equilibrium consists of gases, then the concentrations of all the components can be altered by changing the pressure. When the pressure on the system is increased, the volume decreases proportionately. The total number of moles per unit volume will now be more and the equilibirum will shift in the direction in which there is decrease in number of moles ice., towards the direction in which there is decrease in volume.
Effect of pressure on melting point : There are two types of solids : (a) Solids whose volume decreases on melting, egg., ice, diamond, carborundum, magnesium nitride and quartz Solid (higher volume) Liquid (lower volume) Them process of melting is facilitated at high pressure, thus melting point is lowered. .(b) Solids whose volume increase on melting, e.g., Fe, Cu, Ag, Au, etc.
Solid (lower volume) Liquid (higher volume) In this case the process of melting become difficult at high pressure; thus melting point becomes
high.
(c) Solubility of substances : When solid substance are dissolved in water, either heat is evolved (exothermic) or heat is absorbed (endothermic).
KCI + aq KCl(aq) – heat In such cases, solubility increase with increase in temperature. Consider the case of KOH; when this is dissolved, heat is evolved.
KOH + aq KOH(aq) + heat In such cases, solubility decrease with increase in temperature. (d) Solubility of gases in liquids : When a gas dissolves in liquid, there is decrease in volume. Thus, increase of pressure will favour the dissolution of gas in liquid.
Effect of temperature : Le-Chatelier's principle predicts a system at equilibrium will tend to shift in the endothermic direction when temperature is raised, for then energy is absorbed as heat and the rise in temperature is opposed. Conversely, an equilibrium will shift in the exothermic direction if the temperature is lowered, for then that energy is released and the reduction in temperature is opposed. Van't Hoff equation shows the dependence of equilibrium constant K on temperature as:
Q.
The plot of log K against 1 -- is a straight line with positive slope (K being the equilibrium constant of a reaction), which of the following is then correct ?
In gaseous dissociation reactions, the total mass remains unchanged and the number of moles increases as a result of the reaction. Thus the average molecular weight and hence the vapour density decreases. In other words, the volume increases at constant temperature and pressure and hence the vapour density decreases.The relationship between vapour density in the beginning and vapour density at equilibrium can be found as follows:
At constant temperature and constant pressure for a fixed mass of gaseous mixtures,
PV = nRT Volume ∝ number of moles
Where Do and D are the vapour densities in the beginning and at equilibrium and no and n are the number of moles in the beginning and at equilibrium.The vapour density of PCI5 at 200°C and 252°C are 70.2 and 57.2 respectively at one atmosphere
Q.
The observation shows that the dissociation constants (KP) for the reaction are
In gaseous dissociation reactions, the total mass remains unchanged and the number of moles increases as a result of the reaction. Thus the average molecular weight and hence the vapour density decreases. In other words, the volume increases at constant temperature and pressure and hence the vapour density decreases.The relationship between vapour density in the beginning and vapour density at equilibrium can be found as follows:
At constant temperature and constant pressure for a fixed mass of gaseous mixtures,
PV = nRT Volume ∝ number of moles
Where Do and D are the vapour densities in the beginning and at equilibrium and no and n are the number of moles in the beginning and at equilibrium.The vapour density of PCI5 at 200°C and 252°C are 70.2 and 57.2 respectively at one atmosphere
Q.
This observation shows that
In gaseous dissociation reactions, the total mass remains unchanged and the number of moles increases as a result of the reaction. Thus the average molecular weight and hence the vapour density decreases. In other words, the volume increases at constant temperature and pressure and hence the vapour density decreases.The relationship between vapour density in the beginning and vapour density at equilibrium can be found as follows:
At constant temperature and constant pressure for a fixed mass of gaseous mixtures,
PV = nRT Volume ∝ number of moles
Where Do and D are the vapour densities in the beginning and at equilibrium and no and n are the number of moles in the beginning and at equilibrium.The vapour density of PCI5 at 200°C and 252°C are 70.2 and 57.2 respectively at one atmosphere
Q.
From the given data, it can be interpreted that .
The rate of chemical reaction at a particular temperature is proportional to the product of the molar concentration of reactants with each concentration term raised to the power equal to the number of molecules of the respective reactant taking part in the reaction. aA + bB → products, rate of reaction a [Aka [Bib = k [Aka [Bib, where k is the rate constant of the reaction.
EQUILIBRIUM CONSTANT (k) For a general reaction aA + bB <=> cC + dD, forward rate rf = kf [A]a [B]b, backward rate rb = kb [Cicpyi , concentrations of reactants & products at equilibrium are related by
where all the concentrations are expressed in mole/liter.
In the expression of equilibrium constant those components are kept whose concentration changes with time.
If equilibrium is estabilished by taking all the components in the reaction then to predict the direction of reactions we calculate the reaction quotient (Q).
The values of expression
at any time during reaction is called reaction quotient if Q > Kc reaction proceed in backward direction until equilibrium in reached if Q < Kc reaction will proceed in forward direction until equilibrium is established if Q = KC Reaction is at equilibrium
Q.
Initial concentration of 'A’ is twice the initial concentration of `B'. At equilibrium concentration of 'A and 'a are same then equilibrium constant for the reaction is
The rate of chemical reaction at a particular temperature is proportional to the product of the molar concentration of reactants with each concentration term raised to the power equal to the number of molecules of the respective reactant taking part in the reaction. aA + bB → products, rate of reaction a [Aka [Bib = k [Aka [Bib, where k is the rate constant of the reaction.
EQUILIBRIUM CONSTANT (k) For a general reaction aA + bB <=> cC + dD, forward rate rf = kf [A]a [B]b, backward rate rb = kb [Cicpyi , concentrations of reactants & products at equilibrium are related by
where all the concentrations are expressed in mole/liter.
In the expression of equilibrium constant those components are kept whose concentration changes with time.
If equilibrium is estabilished by taking all the components in the reaction then to predict the direction of reactions we calculate the reaction quotient (Q).
The values of expression
at any time during reaction is called reaction quotient if Q > Kc reaction proceed in backward direction until equilibrium in reached if Q < Kc reaction will proceed in forward direction until equilibrium is established if Q = KC Reaction is at equilibrium
q.
Above homogeneous reaction is carried out in a 2 litre container at a particular temperature by taking 1 mole each of A, B, C and D respectively. If Kc for the reaction is then equilibrium concentration of C is
The rate of chemical reaction at a particular temperature is proportional to the product of the molar concentration of reactants with each concentration term raised to the power equal to the number of molecules of the respective reactant taking part in the reaction. aA + bB → products, rate of reaction a [Aka [Bib = k [Aka [Bib, where k is the rate constant of the reaction.
EQUILIBRIUM CONSTANT (k) For a general reaction aA + bB <=> cC + dD, forward rate rf = kf [A]a [B]b, backward rate rb = kb [Cicpyi , concentrations of reactants & products at equilibrium are related by
where all the concentrations are expressed in mole/liter.
In the expression of equilibrium constant those components are kept whose concentration changes with time.
If equilibrium is estabilished by taking all the components in the reaction then to predict the direction of reactions we calculate the reaction quotient (Q).
The values of expression
at any time during reaction is called reaction quotient if Q > Kc reaction proceed in backward direction until equilibrium in reached if Q < Kc reaction will proceed in forward direction until equilibrium is established if Q = KC Reaction is at equilibrium
Q.
When C,H5OH and CH3COOH are mixed in equivalent proportion equilibrium is reached when 2/3 of acid and alcohol are used. How much ester will be present when 2 moles of acid were to react with 2 moles of alcohol? .














