Chemical Kinetics (Chapter Test - Medical) - Class 12 MCQ
20 Questions MCQ Test - Chemical Kinetics (Chapter Test - Medical)
A hypothetical reaction,
A2 + B2 → 2AB follows the following mechanism
I. A2 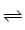 A + A .. (FAST)
A + A .. (FAST)
II. A + B2 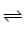 AB + B .. (SLOW)
AB + B .. (SLOW)
III. A + B  AB .. (FAST)
AB .. (FAST)
The order of the overall reaction is
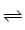 A + A .. (FAST)
A + A .. (FAST)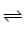 AB + B .. (SLOW)
AB + B .. (SLOW) AB .. (FAST)
AB .. (FAST)The rate law for reaction between the substances A and B is given by
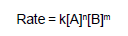
on doubling the concentrations of A and halving the concentration of B, the ratio of the new rate to the earlier
rate of reaction will be
rate of reaction will be
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
For a reaction, A + B→ Products, it is observed that doubling the concentration of B causes the reaction
rate to increases four times, but doubling the concentration of A has no effect on the rate of reaction. The rate
equation is therefore
rate to increases four times, but doubling the concentration of A has no effect on the rate of reaction. The rate
equation is therefore
The following data pertains to reaction between A and
B
Which of the following reference(s) can be drawn from the above data ?
I. Rate constant of the reaction is 1.0 x 10-4
II. Rate law of the reaction is : rate = k [A] [B]
III. Rate of reaction increases four times on
doubling the concentration of both the reactants
Select the correct answer using the codes given below
Which one of the following equation is correct for the reaction,
Units of specific reaction rate for 2nd order reaction is
The units of rate constant and rate of reaction are identical for
Fill in the blanks in the following table for the reaction X + Y → Z . The reaction is of the first order w.r.t. X and
zero order w.r.t. Y.
For a reaction, P +Q → 2R + S . Which of the following
statements is incorrect ?
Match the rate law given in column I with the dimensions of rate constants given in column II and mark the
appropriate choice.
At room temperature, the reaction between NO and O2 to give NO2 is fast, while that between CO and O2 is
slow. It is due to
The rate of equation for the reaction 2A + B→C is found to be : rate = k[A][B]. The correct statement in relation to this reaction is that
A mixture of hydrogen and oxygen gases are indefinitely stable at room temperature but if stuck by a spark the mixture immediately explodes because
The activation energy for the forward reaction A + B ⇔ C + D is 38 kJ and enthalpy of reaction is 20 kJ. The
activation energy for this same reaction in the reverse direction will be
Which of the following simple reactions is trimolecular reaction ?
The half-life period of radioactive materials is 15 minutes. What percent of radioactive of that material
with remain after 45 minutes ?

















