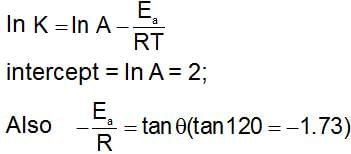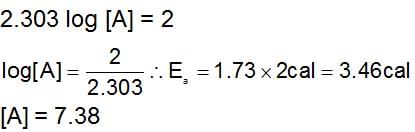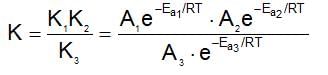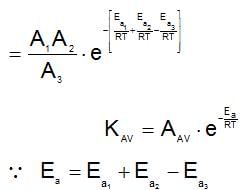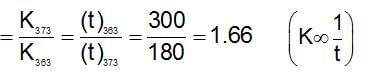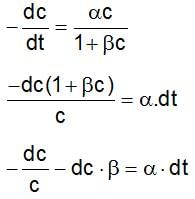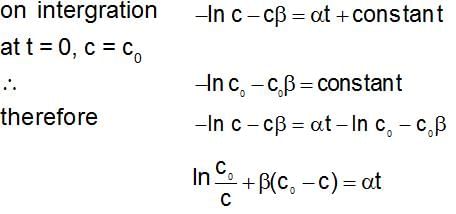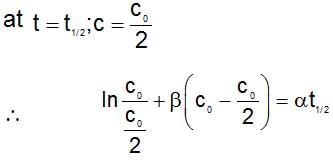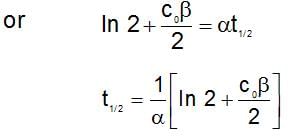Chemical Kinetics - Class 12 MCQ
30 Questions MCQ Test - Chemical Kinetics
In the reaction:A + 2B→ 3C+D ,which of the following expression does not described change in the concentration of various species as a function of time?
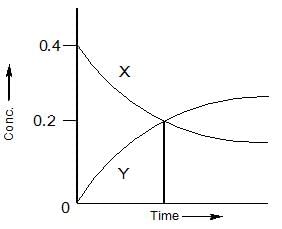
The accompanying figure depicts the change in concentration of species X and Y for the reaction , as a function of time. The point of intersection of the two curves represnts.

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In a reaction, the rate expression is, rate = K [A] [B] 2/3 [C] 0, the order of reaction is:
For a reversible reaction A 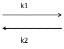 B, Ist order in both the directions, the rate of reaction is given by:
B, Ist order in both the directions, the rate of reaction is given by:
For the decomposition of N2O5(g), it is given that:
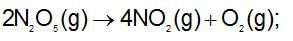
Activation energy Ea
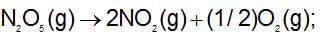
Activation energy E'a
then:
A reaction, occurs by the following mechanisum :
A2 ⇆ A + A .......(Slow)

Its order would be:
The rate of reaction becomes 2 times for every 100C rise in temperature. How the rate of reaction will increase when temperature is increased from 300C to 800C?
A drop of solution (volume 0.05 mL) contains 3.0 x 10-6 mole of H+. If the rate constant of disappearance of H+ is 1.0 x 10-7 mol litre-1 sec-1. How long would it take for H+ in drop to disappear?
Ethylene is produced by cyclobutane as:
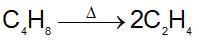
The rate constant is 2.48 x 10-4 sec-1. In what time will the molar ratio of the ethylene to cyclobutane in reaction mixture attain the value equals to unity ?
A reaction takes place in three steps with individual rate constant and activation energy.
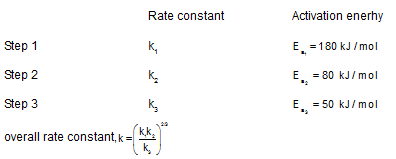
Overall rate constant, K = (k1k3/k2)2/3
Overall activation energy of the reaction will be:
In the following first order competing reactions :
A + Reagent ---> Product
B + Reagent ---> Product
The ratio of K1/K2, if only 50% of B will have been reacted when 94% of A has been reacted in same time is:
From the following data, the activation energy for the reaction (cal/mol):
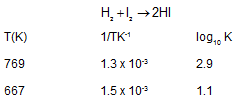
A substance undergoes first order decomposition. The decomposition follows two parallel first order reactions as:
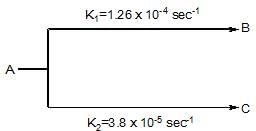
The percentage distribution of B and C are:
Mathematical expression for t1/4, i.e., when (1/4)th reaction is over following first order kinetics can be given by:
The rateconstant of a second order reaction is 10-2 (Ms)-1. The rate constant expressed in cc. molecule-1 min-1 is:
The rate of disappearance of SO2 in the reaction; 2SO2 + O2 ---> 2SO3 is 1.28 x 10-3 g/sec. The the rate of formation of SO3 is:
The rate of a first order reaction is 1.5 x 10-2 M min-1 at 0.5 M concentration of reactant. The half-life of reaction is:
For a reaction aA -->bB when [A] = 2.2 mM, the rate was found to be 2.4 mMs-1. On reducing concentration of [A] to half , the rate changes to 0.6 mMs-1. The order of reaction with respect to A is:
During the kinetic study of the reaction following results were obtained.
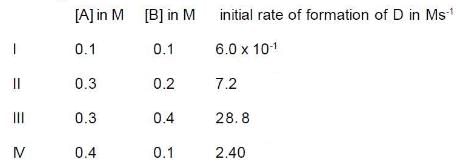
On the basis the data, which one is correct?
The reaction L ---> 4 is started with 10 g of L. After 30 and 90 mintue, 5 g and 1.25 g of L are left respectively. The order of reaction is :
The differential rate expression for the reaction H2 + I2 --> 2HI is:
In a first order reaction the concentration of reactant decreases from 800 mol/dm3 to 50 mol/dm3 in 2 x 104 sec. The rate constant of reaction in sec-1 is:
Plots showing the variation of the rate constant (k) with temperature (T) are given below. The plot that follows Arrhenius equation is:
The rate constants of three reactions involving reactant A only obeying I, II, III order respectively is same. Which of the following is true?
loge K vs. 1/T plots shows a straight line having slope of 1200 and intercepts on the Y-axis of 2, then :
A reaction occurs in three rate detemining steps having rate constant K1, K2 and K3 respectively and arrehenius factor A1, A2, A3 respectively. The overall rate constant 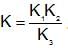 . If energy of activations for each step is Ea1, Ea2, and Ea3 respectively, then overall energy of activation is:
. If energy of activations for each step is Ea1, Ea2, and Ea3 respectively, then overall energy of activation is:
At 3 km altitude, water boils at 900C and 300 seconds are taken to cook a ‘3 minute egg’. The temperature coefficient for the process of cooking is:
The rate expression for a reaction is 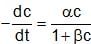 (α and β > 0). The half life of the reaction can be given by:
(α and β > 0). The half life of the reaction can be given by:


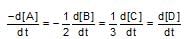
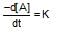
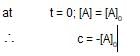
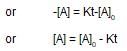
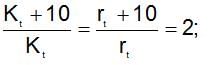
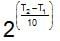 x rate , the rate increases by 25 times, i.e., 32 times.
x rate , the rate increases by 25 times, i.e., 32 times. 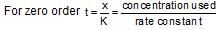
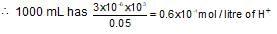
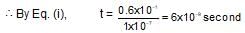
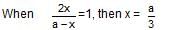
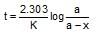
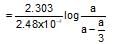
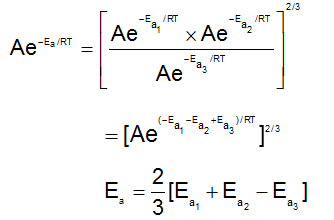
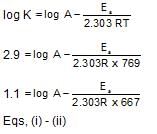
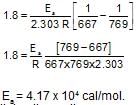
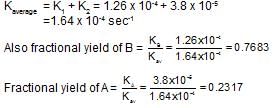
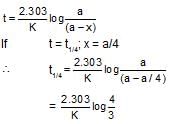
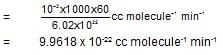
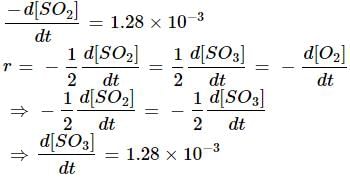

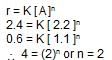
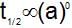 which is obeyed by first order reaction.
which is obeyed by first order reaction.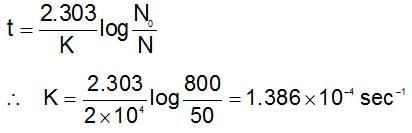
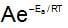 increase exponentially, with rise in temperature.
increase exponentially, with rise in temperature.