Chemical Reactions And Equations - MCQ Test, Class 10 Science - Class 10 MCQ
30 Questions MCQ Test - Chemical Reactions And Equations - MCQ Test, Class 10 Science
Which of the following is not a decomposition reacion?
Which two gases are released on heating lead nitrate?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
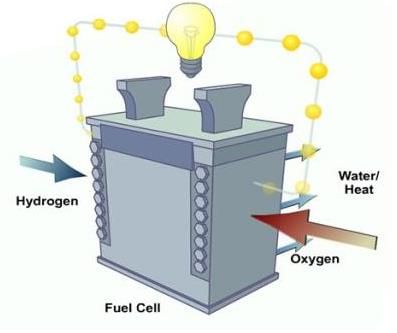
What happens when Hydrogen combines with oxygen in the presence of an electric current?
Which of the statement about the reaction below are incorrect? 2PbO(s)+C(s)→2Pb(s)+CO2(g)
(i) lead is getting reduced,
(ii) Carbon dioxide is getting oxidised,
(iii) Carbon is getting oxidised,
(iv) Lead oxide is getting reduced
Match the following with correct response.
(1) Oxidation (A) Gain of electron
(2) Reduction (B) Electron accepting species
(3) Reducing agent (C) Electron losing species
(4) Oxidising agent (D) Loss of electron
Which of the following represent a double displacement reaction?
Some crystals of copper sulphate were dissolved in water. The colour of the solution obtained would be
Mg ribbon burns with a dazzling flame in air (oxygen) and changes into a white substance, Magnesium oxide. Magnesium is
What happens when dilute HCl is added to iron fillings? Select the correct answer.
Match the following with correct response.
(1) Chemical change (2) Physical change (3) Exothermic (4) Endothermic
(A) Energy releasing (B) Rusting of iron (C) Energy absorbed (D) Dissolution of sugar or salt in water
Which statement is correct about the following reaction?
ZnO + CO Zn + CO2
The colour of zinc metal is
The reactions in which more reactive element can displace less reactive element from a compound are called
Colour of copper sulphate solution changes when an iron nail is dipped in it because of
Match the following with correct response.
(1) Gain of hydrogen (2) Loss of hydrogen (3) Unpleasant taste and foul small in fatty food (4) Eating away of metal in the presence of air and moisture
(A) Reduction (B) Rancidity (C) Oxidation (D) Corrosion
Fe2O3 + 2Al Al2O3 + 2Fe This reaction is an example of –
Which of the following is a decomposition reaction?
Which one of the following will be required to identify the gas evolved when diluted HCl reacts with zinc metal?
Decomposition of ferrous sulphate into Fe2O3,SO2 and SO3 occurs in the presence of
What happens when copper metal is added to silver nitrate solution?
Match the following with correct response.
(1) Reaction in which two substances combine to form new product
(2) substance split to form two or more simple substance
(3) More reactive metal displaces less reactive metal
(4) Loss or gain of electrons occurs
(A) Displacement reaction
(B) Combination Reaction
(C) Redox reaction
(D) Decomposition
When Iron nails are added to an aquous solution of copper sulphate, a chemical change occurs, which of the following is not true about this reaction?
Which of the following reaction is metathesis reaction?
Which of the following is not a balanced chemical equation?
The compound formed by Hg+2 and CI- is
Fe2O3+2Al→Al2O3+2Fe This reaction is an example of -
Match the following with correct response.
(1) Quick lime is added to water (2) Formula for rust (3) Silver tarnishing (4) Pop sound
(A) Ca(OH)2 (B) H2S gas (C) Fe2O3.xH2O (D) H2 gas
Pieces of Zn metal are added to four different test tubes containing different solutions. In which test tube no change is observed?
Can we store CuSO4 in iron container and why?
H2S burns in air to give