Chemical Reactions & Equations - 3 - Class 10 MCQ
10 Questions MCQ Test Extra Documents, Videos & Tests for Class 10 - Chemical Reactions & Equations - 3
Which among the following statement(s) is (are) true?
Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) The formation of silver by decomposition of silver chloride.
(ii) Sublimation of silver chloride.
(iii) Decomposition of chlorine gas from silver chloride.
(iv) Oxidation of silver chloride.
Exposure of silver chloride to sunlight for a long duration turns grey due to
(i) The formation of silver by decomposition of silver chloride.
(ii) Sublimation of silver chloride.
(iii) Decomposition of chlorine gas from silver chloride.
(iv) Oxidation of silver chloride.
Which of the following are combination reactions ?
(i) 2KClO3  2KCl + 3O2
2KCl + 3O2
(ii) MGO + H2O → Mg(OH)2
(iii) 4Al + 3O2 → 2Al2O3
(iv) Zn +FeSO4 → ZnSO4 +Fe
 2KCl + 3O2
2KCl + 3O2(ii) MGO + H2O → Mg(OH)2
(iii) 4Al + 3O2 → 2Al2O3
(iv) Zn +FeSO4 → ZnSO4 +Fe
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
What is observed when a solution of potassium iodide is added to lead nitrate solution ?
Barium chloride on reacting with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved ?
(i) Displacement reaction
(ii) Precipitation reaction
(iii) Combination reaction
(iv) Double displacement reaction
Which of the following statements about the given reaction are correct ?
3Fe(s) + 4H2O(g) → Fe3O4(g) + 6H2 (g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as an oxidising agent
Which of the following is not a decomposition reaction ?
Which of the following reactions will not take place?
Oxidation involves
(i) gain of electron
(ii) loss of electron
(iii) addition of oxygen or electronegative element
(iv) removal of hydrogen or electropositive element
In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
|
5 videos|292 docs|59 tests
|
|
5 videos|292 docs|59 tests
|


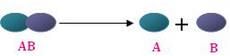 A decomposition reaction occurs when an energy is supplied to a compound causing it to decompose (break down) into multiple different chemical substances.
A decomposition reaction occurs when an energy is supplied to a compound causing it to decompose (break down) into multiple different chemical substances.














