Chemistry- CY 2012 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemistry- CY 2012 GATE Paper (Practice Test)
Q. 1 – Q. 5 carry one mark each.
Q.
If (1.001)1259 = 3.52 and (1.001)2062 = 7.85, then (1.001)3321 =
One of the parts (A, B, C, D) in the sentence given below contains an ERROR. Which one of the
following is INCORRECT?
I requested that he should be given the driving test today instead of tomorrow.
following is INCORRECT?
I requested that he should be given the driving test today instead of tomorrow.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following options is the closest in meaning to the word given below?
Latitude
Latitude
Choose the most appropriate word from the options given below to complete the following
sentence:
Given the seriousness of the situation that he had to face, his ___ was impressive.
Choose the most appropriate alternative from the options given below to complete the following
sentence:
If the tired soldier wanted to lie down, he ___ the mattress out on the balcony.
Q. 6 - Q. 10 carry two marks each.
Q
One of the legacies of the Roman legions was discipline. In the legions, military law prevailed
and discipline was brutal. Discipline on the battlefield kept units obedient, intact and fighting,
even when the odds and conditions were against them.
Which one of the following statements best sums up the meaning of the above passage?
A and B are friends. They decide to meet between 1 PM and 2 PM on a given day. There is a
condition that whoever arrives first will not wait for the other for more than 15 minutes. The
probability that they will meet on that day is
The data given in the following table summarizes the monthly budget of an average household.
The approximate percentage of the monthly budget NOT spent on savings is
There are eight bags of rice looking alike, seven of which have equal weight and one is slightly
heavier. The weighing balance is of unlimited capacity. Using this balance, the minimum number
of weighings required to identify the heavier bag is
Raju has 14 currency notes in his pocket consisting of only Rs. 20 notes and Rs. 10 notes. The total
money value of the notes is Rs. 230. The number of Rs. 10 notes that Raju has is
Q. 11 – Q. 35 carry one mark each
Q.
In the proton decoupled 13C NMR spectrum of 7-norbornanone, the number of signals obtained is
I dentify the most probable product in the given reaction
I n the cyclization reaction given below, the most probable product formed is
If Δy and Δpy are the uncertainties in the y-coordinate and the y component of the momentum of a
particle respectively, then, according to uncertainty principle ΔyΔpy is ( h=2/2π) and h is Planck’s
constant)
The average length of a typical α-helix comprised of 10 amino acids is
Number of thymine residues in a 5000 kb DNA containing 23% guanine residues is
S hown below is a Hammett plot obtained for the reaction
The change in slope of the plot indicates that
The ratio of relative intensities of the two molecular ion peaks of methyl bromide (CH3Br) in the
mass spectrum is
A disaccharide that will not give Benedict’s test and will not form osazone is
T he angular part of the wavefunction for the electron in a hydrogen atom is proportional to
. The values of the azimuthal quantum number ( l ) and the magnetic quantum
number (m ) are, respectively
L et and
denote the wavefunctions of the 2px and 2pz orbitals of carbon, respectively, and
and
represent the wavefunctions of the 2px and 2pz orbitals of oxygen, respectively. If c1 and c2 are constants used in linear combinations and the CO molecule is oriented along the z axis, then, according to molecular orbital theory, the π-bonding molecular orbital has a wave function given by
The bond that gives the most intense band in the infrared spectrum for its stretching vibration is
I f xA and xB are the respective mole fractions of A and B in an ideal solution of the two and TA, TB,
T are the fusion temperatures of pure A, pure B and the ideal solution respectively, then
For a reaction involving two steps given below
assume that the first step attains equilibrium rapidly. The rate of formation of P is proportional to
A metal chelate that can be used for separation and quantitative analysis of aluminium ions by gas
chromatography is
The enthalpies of hydration of Ca2+, Mn2+ and Zn2+ follow the order
The number of terminal carbonyl groups present in Fe2(CO)9 is
Among the following substituted silanes, the one that gives cross-linked silicone polymer upon
hydrolysis is
The plot of versus T (where
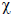 is molar magnetic susceptibility and T is the temperature) for a
is molar magnetic susceptibility and T is the temperature) for a
paramagnetic complex which strictly follows Curie equation is

















