Chemistry- CY 2013 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemistry- CY 2013 GATE Paper (Practice Test)
Q. 1 – Q. 5 carry one mark each.
Q.
if 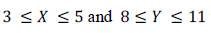 then which of the following options is TRUE
then which of the following options is TRUE
The Headmaster ___________ to speak to you.
Which of the following options is incorrect to complete the above sentence?
Which of the following options is incorrect to complete the above sentence?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Mahatama Gandhi was known for his humility as
"All engineering students" "should learn mechanics", "mathematics and" "how to do computation"
I II III IV
Which of the above underlined parts of the sentence is not appropriate?
Select the pair that best expresses a relationship similar to that expressed in the pair:
water: pipe::
Q. 6 to Q. 10 carry two marks each.
Q.
Velocity of an object fired directly in upward direction is given by V = 80 − 32 t, where t (time)
is in seconds. When will the velocity be between 32 m/sec and 64 m/sec?
In a factory, two machines M1 and M2 manufacture 60% and 40% of the autocomponents
respectively. Out of the total production, 2% of M1 and 3% of M2 are found to be defective. If a
randomly drawn autocomponent from the combined lot is found defective, what is the probability
that it was manufactured by M2?
Following table gives data on tourists from different countries visiting India in the year 2011.
Which two countries contributed to the one third of the total number of tourists who visited India in
2011?
If |−2x + 9| = 3 then the possible value of |−x| − x2would be:
All professors are researchers
Some scientists are professors
Which of the given conclusions is logically valid and is inferred from the above arguments:
Q. 11 – Q.35 carry one mark each.
Q.
The point group symmetry ofCH2=C=CH2 is
Two trial wave functions give ground state
energies E1 and E2, respectively, for themicroscopic particle in a 1-D boxby using the variation
method. If the exact ground state energy is E0, the correct relationship between E0, E1 and E2 is
The ground state energies of H atom and H2 molecule are –13.6 eV and –31.7 eV, respectively. The
dissociation energy of H2 is _______ eV.
A 2 L vessel containing 2 g of H2gas at 27 °C is connected to a 2 L vessel containing 176 g of CO2
gas at 27 °C. Assuming ideal behavior of H2 and CO2, the partial pressure of H2 at equilibrium is
________ bar.
Consider the reaction at equilibrium.
The equilibrium can be shifted towards the forward direction by
A sparingly soluble electrolyte M2X ionizes as
The solubility product (Ksp), molal solubility (S) and mean molal activity coefficient (γ ) are related
by
For the first order consecutive reaction P → Q → R,
under steady state approximation to [Q], the variations of [P], [Q] and [R] with timeare best
represented by
At 273 K and 10 bar,the Langmuir adsorption of a gas on a solid surface gave the fraction of
surface coverage as0.01. The Langmuir adsorption isotherm constant is _________ bar–1.
(Give the answer to the third decimal place)
Conversion of boron trifluoride to tetrafluoroborate accompanies
The correct statement with respect to the bonding of the ligands, Me3N and Me3P with the metal
ions Be2+ and Pd2+ is,
crystal has the lattice parameters a ≠ b ≠ c and α= β= γ= 90o. The crystal system is
The by-product formed in the characteristic reaction of (CO)5Cr=C(OMe)(Me) with MeNH2 is
The catalyst and co-catalyst used in the Wacker process, respectively, are
Oxymyoglobin Mb(O2) and oxyhemoglobinHb(O2)4, respectively, are
Hapticity of cycloheptatrienein Mo(C7H8)(CO)3is _______.
Important : you should answer only the numeric value
The number of oxygen molecule(s) that a molecule of hemerythrin can transport is _______.
Important : you should answer only the numeric value
The maximum number of stereoisomers possible for the compound given below is _____.
Important : you should answer only the numeric value
The correct sequence of the amino acids present in the tripeptide given below is
Among the compounds given in the options A-D, the one that can be used as a formyl anion
equivalent (in the presence of a strong base) is
The major product formed in the reaction given below is

















