Chemistry- CY 2014 GATE Paper (Practice Test) - GATE MCQ
30 Questions MCQ Test - Chemistry- CY 2014 GATE Paper (Practice Test)
Q. 1 – Q. 5 carry one mark each.
Q
A student is required to demonstrate a high level of comprehension of the subject, especially in the
social sciences
The word closest in meaning to comprehension is
social sciences
Choose the most appropriate word from the options given below to complete the following
sentence.
One of his biggest ______ was his ability to forgive.
sentence.
One of his biggest ______ was his ability to forgive.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Rajan was not happy that Sajan decided to do the project on his own. On observing his
unhappiness, Sajan explained to Rajan that he preferred to work independently.
Which one of the statements below is logically valid and can be inferred from the above sentences?
unhappiness, Sajan explained to Rajan that he preferred to work independently.
Which one of the statements below is logically valid and can be inferred from the above sentences?
If y = 5x2 + 3, then the tangent at x = 0, y = 3
A foundry has a fixed daily cost of Rs 50,000 whenever it operates and a variable cost of Rs 800Q,
where Q is the daily production in tonnes. What is the cost of production in Rs per tonne for a daily
production of 100 tonnes?
Important : you should answer only the numeric value
Q. 6 – Q. 10 carry two marks each
Q.
Find the odd one in the following group: ALRVX, EPVZB, ITZDF, OYEIK
Anuj, Bhola, Chandan, Dilip, Eswar and Faisal live on different floors in a six-storeyed building
(the ground floor is numbered 1, the floor above it 2, and so on). Anuj lives on an even-numbered
floor. Bhola does not live on an odd numbered floor. Chandan does not live on any of the floors
below Faisal’s floor. Dilip does not live on floor number 2. Eswar does not live on a floor
immediately above or immediately below Bhola. Faisal lives three floors above Dilip. Which of the
following floor-person combinations is correct?
The smallest angle of a triangle is equal to two thirds of the smallest angle of a quadrilateral. The
ratio between the angles of the quadrilateral is 3:4:5:6. The largest angle of the triangle is twice its
smallest angle. What is the sum, in degrees, of the second largest angle of the triangle and the
largest angle of the quadrilateral?
Important : you should answer only the numeric value
One percent of the people of country X are taller than 6 ft. Two percent of the people of country Y
are taller than 6 ft. There are thrice as many people in country X as in country Y. Taking both
countries together, what is the percentage of people taller than 6 ft?
The monthly rainfall chart based on 50 years of rainfall in Agra is shown in the following figure.
Which of the following are true? (k percentile is the value such that k percent of the data fall below
that value)
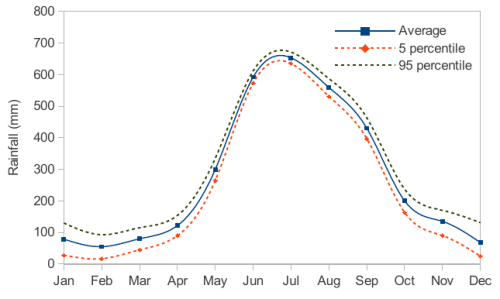
(i) On average, it rains more in July than in December
(ii) Every year, the amount of rainfall in August is more than that in January
(iii) July rainfall can be estimated with better confidence than February rainfall
(iv) In August, there is at least 500 mm of rainfall
Q. 11 – Q. 35 carry one mark each.
Q.
The maximum non-PV work that a system can perform at constant P is
Consider the reaction:
A + B⇔ C
The unit of the thermodynamic equilibrium constant for the reaction is
The number of IR active vibrational normal modes of CO2 is _____________
Important : you should answer only the numeric value
The number of C2 axes in CCl4 is ______________
Important : you should answer only the numeric value
The value of the magnetic quantum number of a px orbital is
The molecular partition function for a system in which the energy levels are equispaced by ε , is
A monoatomic gas, X, adsorbed on a surface, follows Langmuir adsorption isotherm. A plot of the
fraction of surface coverage, θ, against the concentration of the gas [X], for VERY LOW
concentration of the gas, is described by the equation
At a given temperature and pressure, the ratio of the average speed of hydrogen gas to that of
helium gas is approximately ______________
An example of nido-borane from the following is
The geometries of Ni(CO)4 and [NiCl4]2−, respectively, are
The number of S−S bonds in H2S5O6 is ______________
Important : you should answer only the numeric value
In atomic absorption spectroscopy, the atomization process utilizes
At room temperature, the number of singlet resonances observed in the 1H NMR spectrum of
Me3CC(O)NMe2 (N,N-dimethyl pivalamide) is ______________
Important : you should answer only the numeric value
Amongst the following, the metal that does NOT form homoleptic polynuclear metal carbonyl is
The complexes [Co(H2O)4Cl2]NO2 and [Co(H2O)4Cl(NO2)]Cl are
The major product of the following reaction is
Amongst the following, the structure of guanosine is
The correct order of IR stretching frequency of the C=C in the following olefins is
The correct order of the rate of solvolysis for the following chlorides in acetic acid is

















