Chemistry Mock Test - NEET MCQ
30 Questions MCQ Test Additional Study Material for NEET - Chemistry Mock Test
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
50 ml of 1 M oxalic acid is shaken with 0.5 g wood charcoal. The final concentration of the solution after adsorption is 0.5 M. What is the amount of oxalic acid absorbed per gm of carbon?
If same amount of electricity is passed through aqueous solution of AgNO3 and CuSO4 and the number of Ag and Cu atoms deposited are x and y respectively. Then
The e.m.f of the following Daniell cell at 298 k is E1 Zn |ZnSO4(0.01M)| CuSO4(1.0M)| Cu When the concentration of ZnSO4 is 1.0 M and that of CuSO4 is 0.01 M, the e.m.f changed to E2. what is the relationship between E1 and E2 ?
In the metallurgy of iron, when limestone is added to the blast furnace, the calcium ion ends up in
In acidic medium, KMnO4 oxidises FeSO4 solution. Which of the following statement is correct?
The decomposition of a substance follows first order kinetics. Its concentration is reduced to 1/8th of its initial value in 24 minutes. The rate constant of the decomposition process is
For the reaction system 2NO(g) + O2 (g) → 2NO2 (g) the volume is suddenly reduced to half its value by increasing the pressure on it. If the reaction is of first order with respect to O2 and second order with respect to NO, the rate of reaction will be
The freezing point of 0.1 M solution of glucose is -1.860C. If an equal volume of 0.3 M glucose solution is added, the freezing point of the mixture will be
The vapour pressure of benzene at a certain temp. is 640 mm Hg. A non-volatile-non-electrolyte and weighing 2.175g is added to 39.0g of benzene. The vapour pressure of the solution is 600 mm Hg. What is the molecular weight of the solid substance ?
In the laboratory, sodium chloride is made by burning sodium in the atmosphere of chlorine. The salt obtained is yellow in colour. The cause of yellow colour is
In a solid AB having the NaCl structure, ‘A’ atoms occupy the corners of the cubic unit cell. If all the face-centred atoms along one of the axes are removed, then the resultant stoichiometry of the solid is
The products A and B in the following sequence of reactions
R1 — CH2NO2 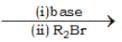 A
A 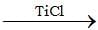 B
B
Respectively are:
An organic compound ‘A’ having molecular formula C2H3N on reduction gave another compound ‘B’. Upon treatment with nitrous acid, ‘B’ gave ethyl alcohol. On warming with chloroform and alcoholic KOH, it formed an offensive smelling compound ‘C’. The compound ‘C’ is
The missing product x in the following reaction will be
3SiF4 + 4H2O → Si(OH)4 + x
A radioactive sample has half life of 1500 years. A sealed tube containing 1 g of a sample will contain g of the sample after 3000 years.The missing figure is
An aqueous solution of sodium sulphate is electrolysed using inert electrodes. The products at the cathode and anode are respectively
Which of the following statements is false for alkali metals ?
Which of the following volume (V)- temperature (T) plots represents the behaviour of one mole of an ideal gas at one atmospheric pressure?
Two glass bulbs A and B are connected by very small tube having a stop cock. Bulb A has a volume of 100 ml and contained the gas while bulb B was empty and had a volume of 150 ml. On opening the stop-cock, the pressure of the gas in bulb A will fall down to
Equal volumes of two gases which do not react together are enclosed in separate vessels. Their pressures at 100 mm and 400 mm respectively. If the two vessels are joined together, then what will be the pressure of the resulting mixture (temperature remaining constant)?
If the nitrogen atom had electronic configuration 1s7, it would have energy lower than that of the normal ground state configuration1s2 2s2 2p3 because the electrons would be closer to the nucleus. Yet 1s is not observed. It violates
What transition in the hydrogen spectrum would have the same wavelength as the Balmer transition, n = 4 to n = 2 in the He+ spectrum?
An element (X) forms compounds of the formula XCl3,X2O5 and Ca3X2 but does not form XCl5. Which of the following is the element X ?
|
26 videos|287 docs|64 tests
|
|
26 videos|287 docs|64 tests
|


 (CH3)2C—O—CH3
(CH3)2C—O—CH3 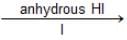 A(mix)
A(mix)














