Chemistry Test - 6 - MCQ
30 Questions MCQ Test - Chemistry Test - 6
In the Cannizzaro’s reaction given below
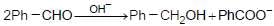
the slowest step isthe slowest step is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Among the following four compounds
(I) Phenol (II) p-Methylphenol
(III) m-Nitrophenol (IV) p-Nitrophenol
The acidity order is
A yellow precipitate is obtained when aqueous AgNO3 is added to a solution of the compound
Which of the following aldehydes will not show Cannizzaro reaction ?
Which one of the following would be expected to be most ionised in water ?
The number of stereoisomers of the compound CH3-CH=CH-CHBrCH3 would be
A compound (A) of formula C3H6Cl2 on reaction with alkali can give (B) of formula C3H6O or (C) of formula C3H4 . (B) on oxidation gave a compound of the formula C3H6O2 . (C) with dilute H2SO4 containing Hg2+ ion gave (D) of formula C3H6O ,which with bromine and NaOH gave the sodium salt of C2H4O2 . Then (A) is
The maximum reactivity of (A) will be when X is ....., for the reaction given below
Cyclohexanone is subjected to reduction by NaBH4 . The product formed is
The correct order of increasing acid strength of the compounds.
I. CH3CO2H II. MeOCH2CO2H
III. CF3CO2H IV.
Consider the following halides
With aq. HCOOH, in SN1 reaction, the decreasing rate of reaction is
Reaction of trans 2-phenyl-1-bromocyclo-pentane on reaction with alcoholic KOH produces
The reaction of chloroform with alcoholic KOH and p-toluidine forms
Which reagent is more effective to convert but-2-enal to but-2-enol ?

















