Chemistry: Topic-wise Test- 4 - NEET MCQ
30 Questions MCQ Test NEET Mock Test Series 2025 - Chemistry: Topic-wise Test- 4
(T) imparts violet colour  (V) Red gas
(V) Red gas  (W) Red ppt.
(W) Red ppt.

(W) Red ppt.  white ppt.
white ppt.
(U)  gas (gives white fumes with HCl)
gas (gives white fumes with HCl)
sublimes on heating Identify (T) to (Z).
The number of moles of acidified KMnO4 required to convert one mole of sulphite ion into sulphate ion is-
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
N2(g) + 3H2 (g)  2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -
 2NH3(g) ; Haber's process, Mo is used as -
2NH3(g) ; Haber's process, Mo is used as -Potash alum is a double salt, its aqueous solution shows the characteristics of-
The structure of the product formed in the reaction given below is -
Vapour pressure of pure water at 298 K is 23.8 torr. Thus, vapour pressure of a solution containing 6 g urea and 68.4 g sucrose in 10 moles of water is
Vapour pressure of nitrobenzene (molar mass = 123 g mol-1) is 3.6 kNm-2 and that of water (molar mass = 18 g mol-1) is 977 kNm-2. They form immiscible mixture at the given temperature. Thus, percentage of nitrobenzene in the vapour phase is
At 298 K, the vapour pressure of pure water is 23.76 mm Hg and that of an aqueous solution is 22.98 mm Hg. Thus, molality of solution is (assume dilute solution)
Elevation in boiling point of a molar (1 M) glucose solution (d = 1.2 g mL-1) is
Relative lowering of vapour pressure of a non-volatile solute X in water is 0.05. If Kb(H20) = 0.52° mol-1 kg-1.
(ΔTb) = elevation in boiling point assuming a dilute solution
(ATb)' = elevation in boiling point assuming a solution of moderate concentration then, {ΔTb) - {ΔTb)' =
Which has maximum osmotic pressure at temperature T K?
There is formation of Prussian blue when Fe3+ reacts with [Fe(CN)6]4-. Two solutions as shown are separated by a semipermeable membrane AB. Due to osmosis, there is
Three solutions of following in water are isothermal conditions as given:
Thus,
Only One Option Correct Type
This section contains 7 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
Q.
The degree of dissociation (α ) o f weak electrolyte AxBy is related to van’t Hoff factor (i) by the expression
[AIEEE 2011]
0.004 M Na2S04 aqueous solution is isotonic with 0.01 M glucose solution at 300 K. Thus, degree of dissociation of Na2S04 is
When 20 g of naphthoic acid (C11H8O2) is dissolved in 50 g of benzene, a freezing point depression of 2 K is observed. [Kf (benzene) = 1.72Kmol-1 kg]. The van’t Hoff factor (i) is
[IIT - JEE 2007]
An aqueous solution of a solute AB has boiling point of 101.08° C and freezes at -1 .80 °C . AB is found to be 100% ionised at boiling point. If Kb /Kf = 0.3, then AB
Among the alkenes which one produces tertiary butyl alcohol on acid hydration
What is the correct increasing order of acidity of the following?
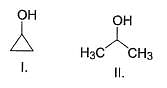
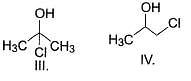
Which of the following can be used for the distinction of ethanol from phenol?
Consider the following reaction,
The above reaction can best be brought about by
Passage III
When suspected drunk drivers are tested with a breathalyser, the alcohol (ethanol) in the exhaled breath is oxidised to ethanoic acid with an acidic solution of potassium dichromate.
Q.
Under standard state, E°cell of the breathalyser would be
Passage III
When suspected drunk drivers are tested with a breathalyser, the alcohol (ethanol) in the exhaled breath is oxidised to ethanoic acid with an acidic solution of potassium dichromate.
Q.
Under the condition of unit activity of each species,emf recorded in the breathalyser at pH 4 would be
E° values of Mn+ + ne- → M are given below
Fe2+/Fe -0.44 V, Mg2+/Mg -2.37 V, Pt(H2)/H+ 0.00 V,
AI3+/AI -1.66 V , Zn2+/Zn - 0.76 V, Cu2+/Cu +0.34 V,
Ag+/Ag + 0.80 V, Ni2+/Ni -0.26 V
How many of the metals can be used to prevent corrosion of Ni to Ni2+?
A fully charged battery contains 500 mL of 5.00 M H2SO4. What is the concentration of H2SO4 in the battery after 6.0 A of current is drawn from the battery for 13.40 h?
A hydrogen-oxygen fuel cell opeartes on the simple reaction:
If the cell is designed to produce 1.46A of current and if the H2 is contained in a 1.0 L tank at 2.00 atm pressure at 298K, how long can the fuel cell operate(in hour) before the H2 runs out.(Assume unlimited supply of oxygen)
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Four electrolytic cells containing solution of ZnSO4, AgNO3, CuSO4 and dil. H2SO4 are connected in series. A steady current of 1.5 A was passed through them until 77 mL of O2 gas at STP is formed in anode compartment of H2SO4 solution. Match the different elements/gas formed In Column I with their values in Column II and select the answer from the codes given below. (Cu = 63.5, Zn = 65, Ag = 108, S = 32, H = 1, 0 = 16)
Codes i ii iii iv
|
1 videos|26 docs|111 tests
|
|
1 videos|26 docs|111 tests
|