First Law Of Thermodynamics MCQ Level – 1 (part - 2) - Physics MCQ
10 Questions MCQ Test - First Law Of Thermodynamics MCQ Level – 1 (part - 2)
If W is the work done by a system against its surrounding, what will –W stand for ?
Select one:
Select one:
Internal energy of a perfect gas depends on.
Select one:
Select one:
In a cyclic process.
Select one:
Select one:
Which of the following is not a property of the system?
Select one:
If a system A is in thermal equilibrium separately with B and C, then B and C are also in thermal equilibrium with each other. This is the statement for.
Select one:
The processes or systems that do not involve heat is called.
Select one:
Let ΔWi = amount of work done by the gas when compressed isothermally to volume V, ΔQ = amount of heat absorbed by the gas during the process, ΔWa = amount of work done by the gas when expanded adiabatically to volume V, ΔE = change in internal energy of the gas due to complete process. So ΔE is given by.
Select one:
First law of the thermodynamics is conservation of.
Select one:
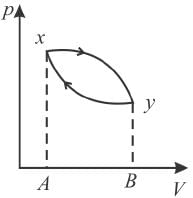
The piston containing an ideal gas is originally is the state x (see figure). The gas is taken through a thermal cycle  as shown.
as shown.
The work done by the gas is positive, if the direction of the thermal cycle is
Select one:
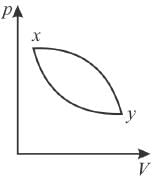
In the pV diagram below, which is the correct statement?
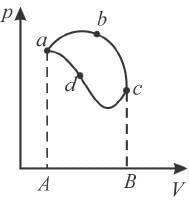
Select one: