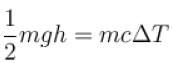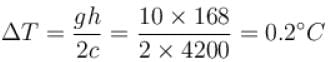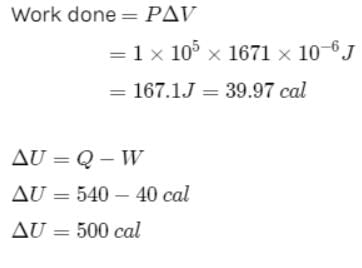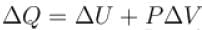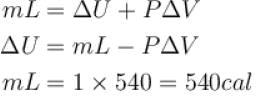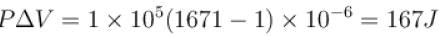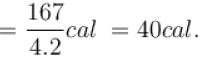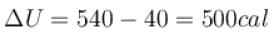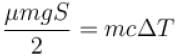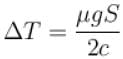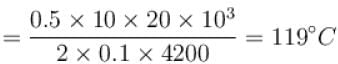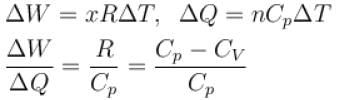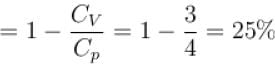First Law Of Thermodynamics MCQ Level – 2 (Part - 1) - Physics MCQ
10 Questions MCQ Test Topic wise Tests for IIT JAM Physics - First Law Of Thermodynamics MCQ Level – 2 (Part - 1)
One mole of an ideal gas expands adiabatically from temperature T1 to temperature T2. The work done by the gas is
Select one:
Select one:
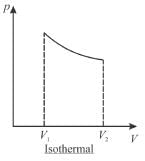
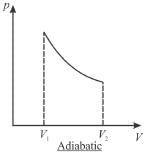
The pressure-volume graph of an ideal gas cycle consisting of isothermal and adiabatic process is shown in the figure. The adiabatic process is described by
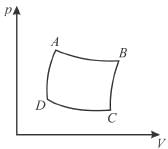
Select one:

Select one:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A water fall is 168m high. Assuming that half the kinetic energy of the falling water gets converted into heat, the rise in temperature of water is approximately (take g = 10m/s2)
Select one:
Select one:
One gram of water on evaporation at atmospheric pressure forms 1671cm3 of steam. Heat of vaporization at this pressure is 540 cal/g. Calculate the increase in the internal energy. (Atmospheric pressure = 1×105N/m2)
Select one:
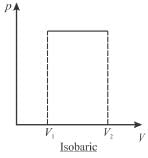
A sample of gas expands from volume V1 to V2. The amount of work done by the gas in greatest when the expansion is.
Select one:
One gram of water on evaporation at atmospheric pressure forms 1671cm3 of steam. Heat of vaporization at this pressure is 540 cal/g. The increase in internal energy is
Select one:
Heat capacity of a substance is infinite. It means :
Select one:
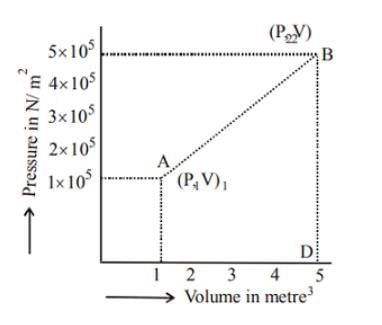
A system changes from the state (p1, V1 ) to the state (p2, V2 ) as shown in the figure. The work done by the system is.
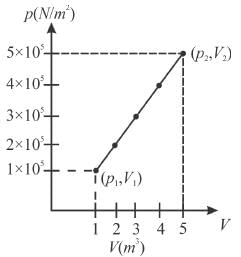
Select one:
A body of mass 25kg is dragged on a rough horizontal road for one hour with a speed of 20km/hr.
If the coefficient of friction is 0.5 and half of the heat produced is absorbed by the body, the rise in its temperature is (specific heat of body = 0.1cal/g ºC, g = 10m/s2)
Select one:
A gas, for which γ is 4/3, is heated at constant pressure. The percentage of heat supplied used for external work.
Select one: