GATE Chemistry Mock Test - 1 - GATE Chemistry MCQ
30 Questions MCQ Test GATE Chemistry Mock Test Series - GATE Chemistry Mock Test - 1
She has a sharp tongue and it can occasionally turn____________:
I __________ made arrangements had I ________informed earlier:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the summer, water consumption is known to decrease overall by 25%. A water board official states that in the summer household consumption decreases by 20% while other consumption increases by 70%
Which of the following statements is correct?
Which of the following statements is correct?
40% of deaths on city roads may be attributed to drunken driving. The number of degrees needed to represent this as a slice of a pie chart is:
Some tables are shelves. Some shelves are chairs. All chairs are benches. Which of the following conclusions can be deduced from the preceding sentences?
(I) At least one bench is a table (II) At least one shelf is a bench
(III) At least one chair is a table (IV) All benches are chairs
“If you are looking for a history of India, or an account of the rise and fall of the British Raj, or for the reason of the cleaving of the subcontinent into two mutually antagonistic parts and the effects this mutilation will have in the respective sections, and ultimately on Asia, you will not find it in these pages; for though I have spent a lifetime in the country. I lived too near the seat of events, and was too intimately associated with the actors, to get the perspective needed for the impartial recording of these matters.” Here, the word ‘antagonistic’ is closest in meaning to:
Trucks (10 m long) and cars (5 m long) go on a single lane bridge. There must be a gap of at least 20 m after each truck and a gap of at least 15 m after each car. Trucks and cars travel at a speed of 36 km/h. If cars and trucks go alternately. What is the maximum number of vehicles that can use the bridge in one hour?
There are 3 Indians and 3 Chinese in a group of 6 people. How many subgroups of this group can we choose so that every subgroup has at least one Indian?
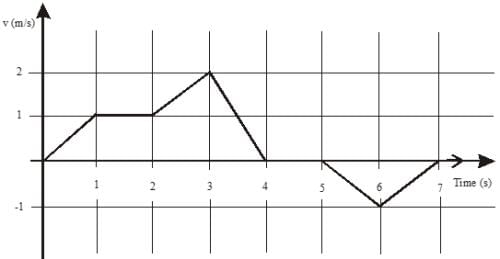
The velocity V of a vehicle along a straight line is measured in m/s and plotted as shown with respect to time in seconds. At the end of the 7 seconds, how much will the odometer reading increase by (in m)?
A cube of side 3 units is formed using a set of smaller cubes of side 1 unit. Find the proportion of the number of faces of the smaller cubes visible to those which are not visible:
Which of the following metallocene has 3.87 BM value of magnetic moment?
Consider the compounds PF5, SbF5, PH3 and SbH3. The strongest acid and strongest base among these are resp:
Which of the following is true for 2px, 2py and 2pz orbitals of a H-atom?
The species which by definition has zero standard molar enthalpy of formation at 298 K is:
In fcc crystal lattice, edge length is 400 pm. Find the diameter of greatest sphere which can be fitted into the interstitial void without distortion of lattice:
The molecules with the smallest rotation partition function at any temperature among the following is:
At room temperature, which molecule has the minimum rotational entropy?
Predict the product for the following sugar functionalization reaction:
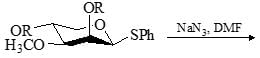
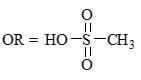
Consider the following:

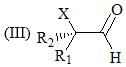
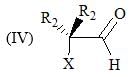
Order of their stretching frequency in IR-spectroscopy would be:
1H NMR spectrum of an organic compound recorded on a 500 MHz spectrometer showed a quartet with line positions at 1759, 1753, 1747, 1741 Hz. Chemical shift (s) and coupling constant (Hz) of the quartet are?
Choose incorrect option in reference to symmetry elements present in ‘Ethane (staggered)’ molecule:
The standard emf of the cell, Cd(s) | CdCl2(aq) (0.1 M) || AgCl(s) | Ag(s) in which the cell reaction, is,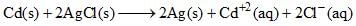 is 0.6915 V at 0°C and 0.6753 V at 25°C. The DH° of the reaction at 25°C is:
is 0.6915 V at 0°C and 0.6753 V at 25°C. The DH° of the reaction at 25°C is:
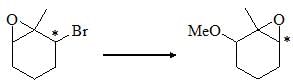
Choose the correct product of the following reaction:
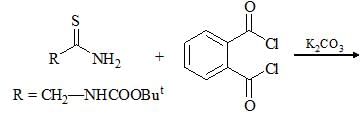
The increasing order of pKa values of the circled hydrogen in the following compounds is?
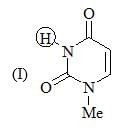
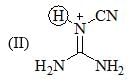
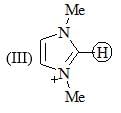
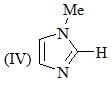
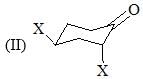
Consider the compound [M(h6-C6H6)(CS)2]2 as a stable compound which follows 18 e– rule. Hence, metal ‘M’ can be
The nodal plane in the π-bond of ethene is located in:
An inventor claims to have constructed an engine that has an efficiency of 75% when operated between the boiling and freezing points of water. Which of the following is true?
Which of the following statement(s) is/are true about the reaction given below?
|
18 docs|37 tests
|
|
18 docs|37 tests
|

















