GATE Chemistry Mock Test - 3 - GATE Chemistry MCQ
30 Questions MCQ Test GATE Chemistry Mock Test Series - GATE Chemistry Mock Test - 3
If (1.001)1259 = 3.52 and (1.001)2062 = 7.85, then (1.001)3321 =?
One of the parts (A, B, C, D) in the sentence given below contains an ERROR. Which one of the following is incorrect? I requested that he should be given the driving test today instead of tomorrow:
Which one of the following options is the closest in meaning to the word given below? Latitude
Choose the most appropriate word from the options given blow to complete the following sentence: Given the seriousness of the situation that he had to face, his ____ was impressive:
Choose the most appropriate alternative from the options given below to complete the following sentence: If the tired soldier wanted to lie, down, he _______ the mattress, out on the balcony.
One of the legacies of the Roman legions was discipline. In the legions, military law prevailed and discipline was brutal. Discipline on the battlefield kept units obedient, intact and fighting, even when the odds and conditions, were against them. Which one of the following statements best sums up the meaning of the above passage?
A and B are friends. They decide to meet between 1 PM and 2 PM on a given day. There is a condition that whoever arrives first will not wait for the other for more than 15 minutes. The probability that they will meet on that day is:
The data given in the following table summarizes the monthly budget of an average household.
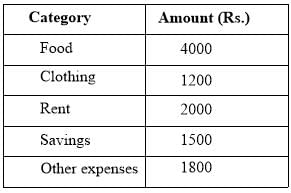
The approximate percentage of the monthly budget not spent on savings is:
There are eight bags of rice looking alike, seven of which have equal weight and one is slightly heavier. The weighing balance is of unlimited capacity. Using this balance, the minimum number of weighing required to identify the heavier bag is:
Raju has 14 currency notes in his pocket consisting of only Rs. 20 notes and Rs. 10 notes. The total money value of the notes is Rs. 230. The number of Rs. 10 notes that Raju has is:
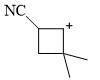
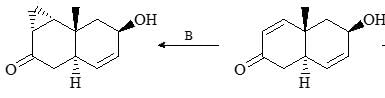
The reagents A and B in the following reactions are:
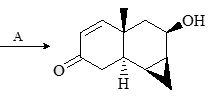
Major product formed in the following chemical reaction would be?
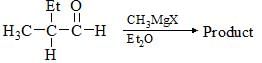
Which of the following chemical conversion is incorrectly presented?
Anhydrous AlCl3 cannot be obtained from which of the following reactions:
Number of nodes in 1-D S.H.O models is given by (V = Vibrational Quantum number):
The percent transmittance of 8 × 10–5 M solution of KMnO4 is 39.8 when measured at 510 nm in a cell of path length of 1 cm. The absorbance and the molar extinction coefficient (in M–1 cm–1) of this solution respectively, are:
Emf of Cd-cell is 1.018 V at 25°C. The temperature coefficient of cell is –5.2 × 10–5 VK–1. How cell temperature will change during operation?
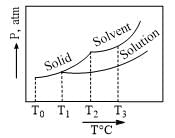
What is the normal freezing point of the solution represented by the phase diagram?
At 30°C the solubility of Ag2CO3 (Ksp = 8 × 10–12) would be greatest in one litre of:
A particle has the position vector r = î - 2ĵ + k and the linear momentum p = 2î - ĵ + k. Its angular momentum about the origin is:
Aluminum metal has a density of 2.72g/cm3 and crystallizes in lattice with an edge length of 404 pm. Which of the following alternative are correct:
Choose correct statemet(s) in reference to fractional distillation:
Which of the following is not a water absorber and dehydrating substance:
In bis(dimethylglyoximato)nickel(II), the number of Ni—N, Ni—O and intramolecular hydrogen bond(s), respectively are:
|
20 docs|37 tests
|