GATE Chemistry Exam > GATE Chemistry Tests > GATE Chemistry Mock Test Series > GOC & Aromaticity - 2 - GATE Chemistry MCQ
GOC & Aromaticity - 2 - GATE Chemistry MCQ
Test Description
20 Questions MCQ Test GATE Chemistry Mock Test Series - GOC & Aromaticity - 2
GOC & Aromaticity - 2 for GATE Chemistry 2024 is part of GATE Chemistry Mock Test Series preparation. The GOC & Aromaticity - 2 questions and answers have been
prepared according to the GATE Chemistry exam syllabus.The GOC & Aromaticity - 2 MCQs are made for GATE Chemistry 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for GOC & Aromaticity - 2 below.
Solutions of GOC & Aromaticity - 2 questions in English are available as part of our GATE Chemistry Mock Test Series for GATE Chemistry & GOC & Aromaticity - 2 solutions in
Hindi for GATE Chemistry Mock Test Series course. Download more important topics, notes, lectures and mock
test series for GATE Chemistry Exam by signing up for free. Attempt GOC & Aromaticity - 2 | 20 questions in 60 minutes | Mock test for GATE Chemistry preparation | Free important questions MCQ to study GATE Chemistry Mock Test Series for GATE Chemistry Exam | Download free PDF with solutions
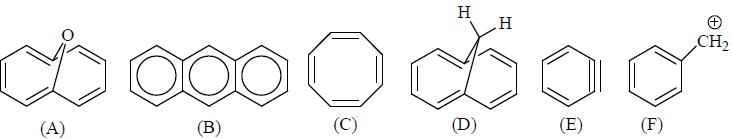
GOC & Aromaticity - 2 - Question 1
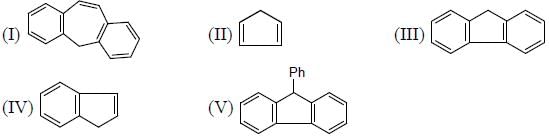
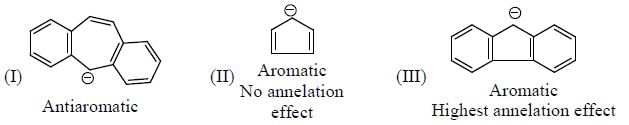
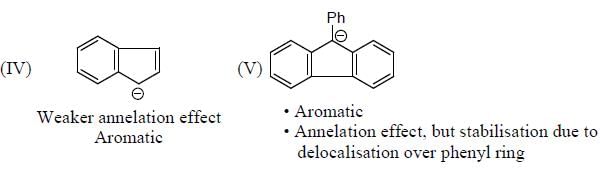
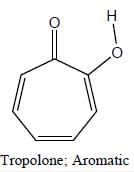
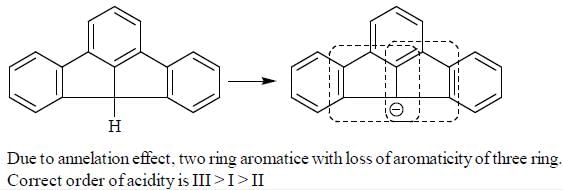
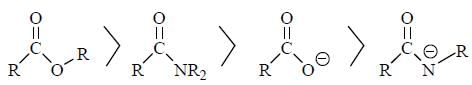
The Correct order of pKa value of the follwing compound will be
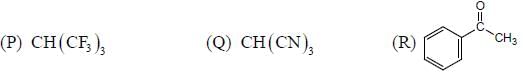

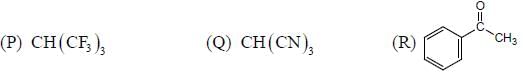

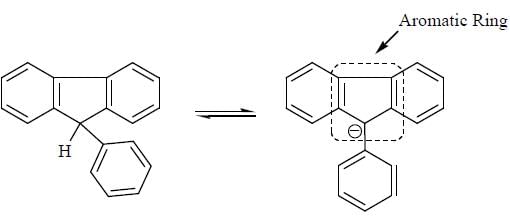
Detailed Solution for GOC & Aromaticity - 2 - Question 1
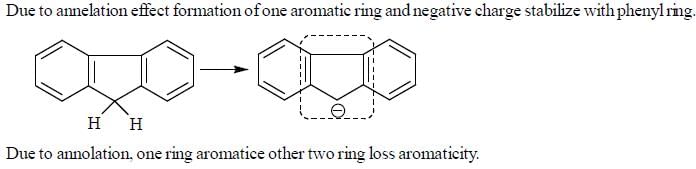
Detailed Solution for GOC & Aromaticity - 2 - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
GOC & Aromaticity - 2 - Question 3
The acidic behaviour of the following substituted benzoic acid will be in the order of
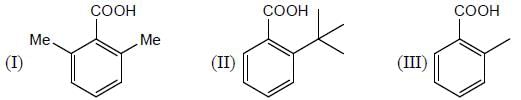

Detailed Solution for GOC & Aromaticity - 2 - Question 3
GOC & Aromaticity - 2 - Question 4
The correct order for the rates of electrophilic aromatic substitution of the following compound is
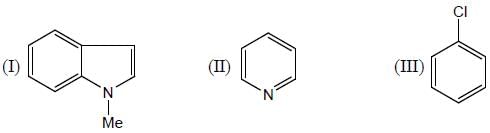
Detailed Solution for GOC & Aromaticity - 2 - Question 4
GOC & Aromaticity - 2 - Question 5
Among the bromide l-lll given below, the order of their reactivity in the SN1 reaction
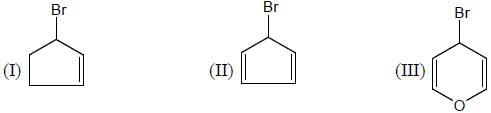
Detailed Solution for GOC & Aromaticity - 2 - Question 5
Detailed Solution for GOC & Aromaticity - 2 - Question 6
Detailed Solution for GOC & Aromaticity - 2 - Question 7
GOC & Aromaticity - 2 - Question 8
The decreasing order ofnucleophilicity for the following anions is
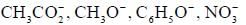
Detailed Solution for GOC & Aromaticity - 2 - Question 8
*Answer can only contain numeric values
GOC & Aromaticity - 2 - Question 9
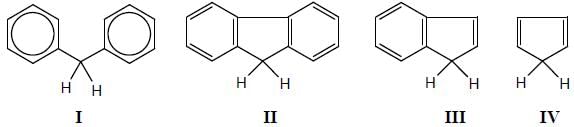
How many compound(s) will not rotate the plane polarized light.
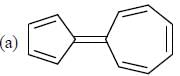
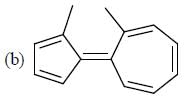
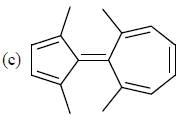
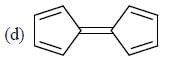
Detailed Solution for GOC & Aromaticity - 2 - Question 9
*Answer can only contain numeric values
Detailed Solution for GOC & Aromaticity - 2 - Question 10
GOC & Aromaticity - 2 - Question 11
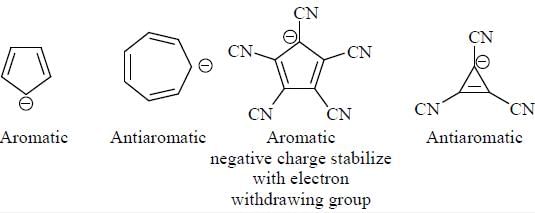
The increasing order of acidity of the following molecules.
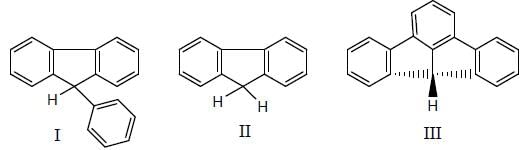
Detailed Solution for GOC & Aromaticity - 2 - Question 11
GOC & Aromaticity - 2 - Question 12
The collect order of increasing reactivity towards nucleophilic addition for the following carbonyl derivative.
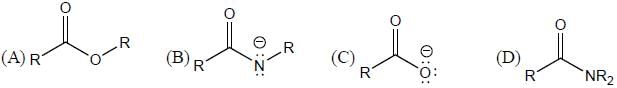
Detailed Solution for GOC & Aromaticity - 2 - Question 12
Detailed Solution for GOC & Aromaticity - 2 - Question 13
Detailed Solution for GOC & Aromaticity - 2 - Question 14
Detailed Solution for GOC & Aromaticity - 2 - Question 15
Detailed Solution for GOC & Aromaticity - 2 - Question 16
Detailed Solution for GOC & Aromaticity - 2 - Question 17
Detailed Solution for GOC & Aromaticity - 2 - Question 18
*Answer can only contain numeric values
GOC & Aromaticity - 2 - Question 19
x = number of (+M) group attached with phenyl ring, so the value of x is
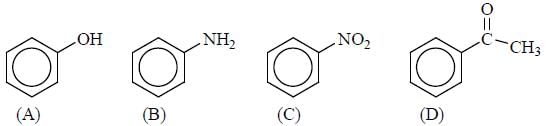
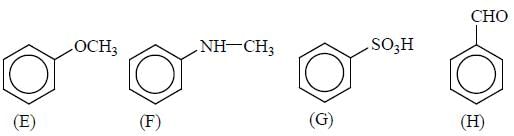
Detailed Solution for GOC & Aromaticity - 2 - Question 19
*Answer can only contain numeric values
Detailed Solution for GOC & Aromaticity - 2 - Question 20
|
18 docs|37 tests
|
Information about GOC & Aromaticity - 2 Page
In this test you can find the Exam questions for GOC & Aromaticity - 2 solved & explained in the simplest way possible.
Besides giving Questions and answers for GOC & Aromaticity - 2, EduRev gives you an ample number of Online tests for practice
|
18 docs|37 tests
|
Download as PDF


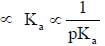
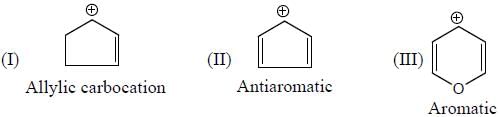

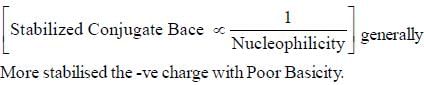
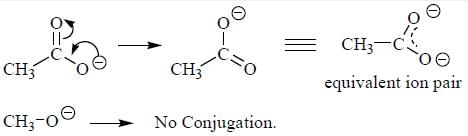
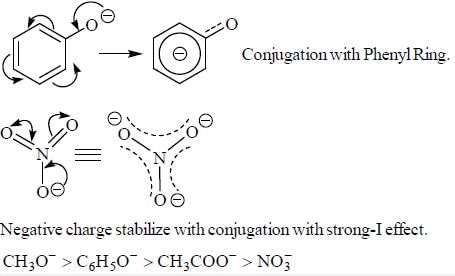
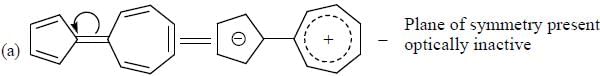
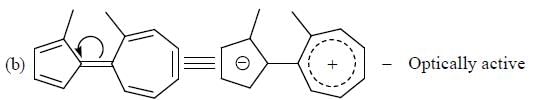
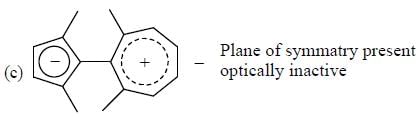

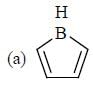
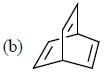
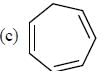
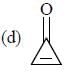
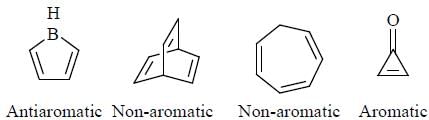
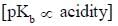
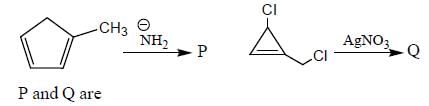
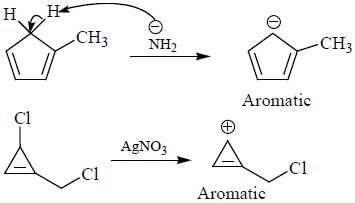
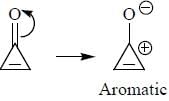
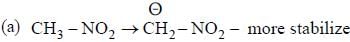
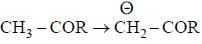
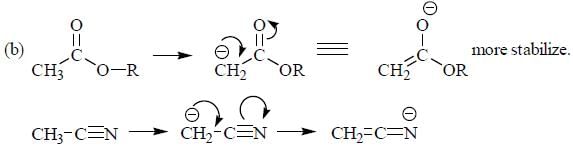
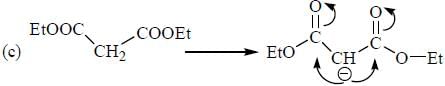
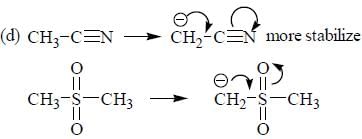
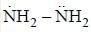 having lower activation energy so they act as more nucleophile.
having lower activation energy so they act as more nucleophile.