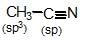Class 12 Exam > Class 12 Tests > GOC - Class 12 MCQ
GOC - Class 12 MCQ
Test Description
30 Questions MCQ Test - GOC
GOC for Class 12 2024 is part of Class 12 preparation. The GOC questions and answers have been prepared
according to the Class 12 exam syllabus.The GOC MCQs are made for Class 12 2024 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for GOC below.
Solutions of GOC questions in English are available as part of our course for Class 12 & GOC solutions in
Hindi for Class 12 course.
Download more important topics, notes, lectures and mock test series for Class 12 Exam by signing up for free. Attempt GOC | 30 questions in 45 minutes | Mock test for Class 12 preparation | Free important questions MCQ to study for Class 12 Exam | Download free PDF with solutions
Detailed Solution for GOC - Question 1
Detailed Solution for GOC - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for GOC - Question 3
GOC - Question 4
Consider the following statements :
(a)  is more stable than
is more stable than 
(b) 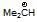 is more stable than
is more stable than 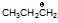
(c) 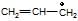 is more stable than
is more stable than 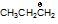
(d) 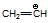 is more stable than
is more stable than 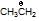
of these statements :
Detailed Solution for GOC - Question 4
Detailed Solution for GOC - Question 5
Detailed Solution for GOC - Question 6
Detailed Solution for GOC - Question 7
GOC - Question 8
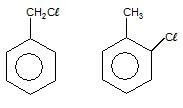
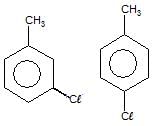
The total number of benzene derivatives having the molecular formula C7H7CI is :
Detailed Solution for GOC - Question 8
Detailed Solution for GOC - Question 9
Detailed Solution for GOC - Question 10
Detailed Solution for GOC - Question 11
Detailed Solution for GOC - Question 12
Detailed Solution for GOC - Question 13
Detailed Solution for GOC - Question 14
GOC - Question 16
Increasing order of stability among the three main conformations (i.e., Eclipse, Anti and Gauche) of 2-fluoroethanol is:
Detailed Solution for GOC - Question 16
Detailed Solution for GOC - Question 17
GOC - Question 18
In the Newman projection for 2,2-dimethylbutane :
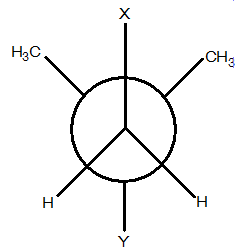
X and Y can respectively be:
Detailed Solution for GOC - Question 18
GOC - Question 19
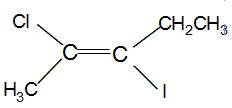
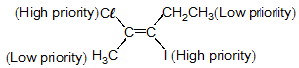
Which of the following is correct set of physical properties of the geometrical isomers?
 and
and 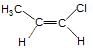
Detailed Solution for GOC - Question 19
Detailed Solution for GOC - Question 20
Detailed Solution for GOC - Question 21
Detailed Solution for GOC - Question 22
Detailed Solution for GOC - Question 23
Detailed Solution for GOC - Question 24
Detailed Solution for GOC - Question 25
Detailed Solution for GOC - Question 26
Detailed Solution for GOC - Question 27
Detailed Solution for GOC - Question 28
Detailed Solution for GOC - Question 29
Detailed Solution for GOC - Question 30
Information about GOC Page
In this test you can find the Exam questions for GOC solved & explained in the simplest way possible.
Besides giving Questions and answers for GOC, EduRev gives you an ample number of Online tests for practice
Download as PDF


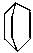 is known by which of the following names?
is known by which of the following names?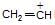 sp hybridised whereas in
sp hybridised whereas in 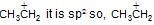 is more stable than
is more stable than 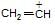
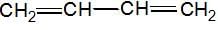
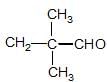 has II— polar bond but does not have bond ∝-Hydrogen.
has II— polar bond but does not have bond ∝-Hydrogen.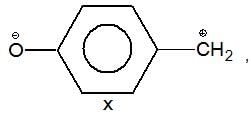
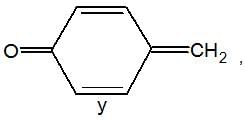

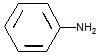 is a weaker base due to -R-effect of benzene
is a weaker base due to -R-effect of benzene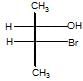
 configuration is studied around C-2 & C-3
configuration is studied around C-2 & C-3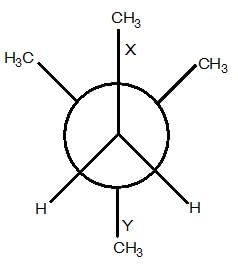
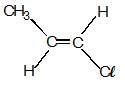
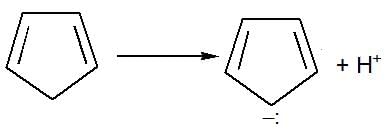
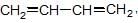 each C-atom 3σ— has bonds, so, they are sp2 hybridised.
each C-atom 3σ— has bonds, so, they are sp2 hybridised.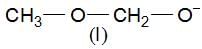
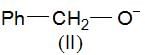
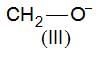
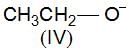
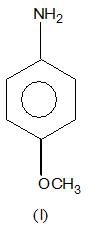

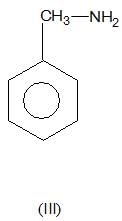
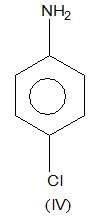
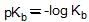 , higher pKb means weaker base
, higher pKb means weaker base