Halogen Derivatives - Class 12 MCQ
30 Questions MCQ Test - Halogen Derivatives
Haloalkanes contain halogen atom(s) attached to the sp3 hybridesed carbon atom of an alkyl group. Identify haloalkane from the following compounds?
(i) 2-bromopentane (ii) vinyl chloride (chloroethane)
(iii) 2-chloroacetophenone (iv) trichloromethane
(i) 2-bromopentane (ii) vinyl chloride (chloroethane)
(iii) 2-chloroacetophenone (iv) trichloromethane
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In 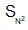 reactions the sequence of bond breaking and bond formation is as follows:
reactions the sequence of bond breaking and bond formation is as follows:
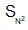 reactions the sequence of bond breaking and bond formation is as follows:
reactions the sequence of bond breaking and bond formation is as follows: Which of the following alkyl halides is hydrolysed by 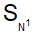 mechanism?
mechanism?
Arrange the following compounds in decreasing order of their boiling points
(i) CH3Br (ii) CH3CH2Br (iii) CH3CH2CH2Br (iv) BrC6H4Br
Arrange the following compounds in order of their reactivity towards 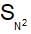 reaction
reaction
(i)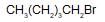
(ii)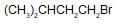
(iii)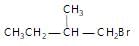
(iv)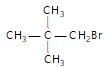
The compound which undergoes fastest reaction with aq. NaOH solution is:
Consider the following reaction

Which of the following products is not expected to form?
Which of the following reactions will undergo an elimination reaction and an alkene will be formed in the product?
When 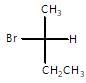 reacts with KOH in presence of water (through SN2 reaction mechanism) than stereochemistry of product so formed will be:
reacts with KOH in presence of water (through SN2 reaction mechanism) than stereochemistry of product so formed will be:
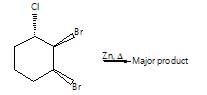
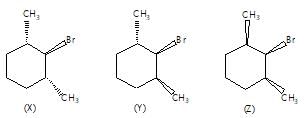
Which among the following on dehalogenation will give trans-alkene?
In the following compounds, nucleophile and the leaving group are in the same molecule:
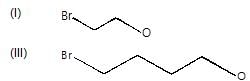
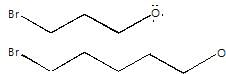
These dual nature species can undergo intermolecular and intramolecular nucleophilic substitution. Intramolecular substitution reaction is possible in:
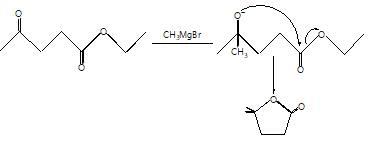
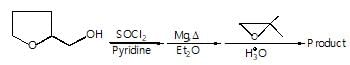
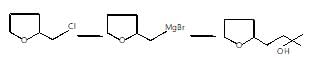
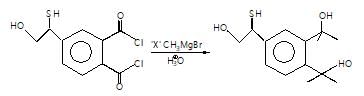
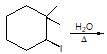
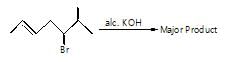
The major organic products ‘A’ and ‘B’ in the given reactions are respectively :
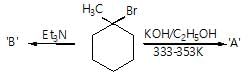
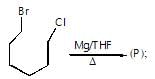
Only one of the following alkyl halides can be prepared as the major product of the addition of HBr to an alkene:


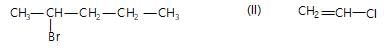

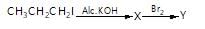
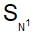 reaction are governed by stability of carbocation, so, 30 halides are more reactive
reaction are governed by stability of carbocation, so, 30 halides are more reactive reactions decreases with increase in steric hindrance i.e crowding or branching around B-carbon
reactions decreases with increase in steric hindrance i.e crowding or branching around B-carbon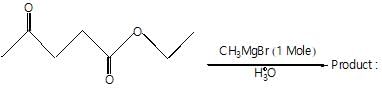
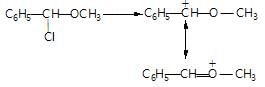
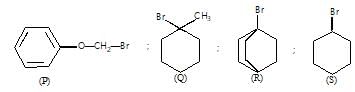
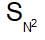 where elimination competes substitution, it shows inversion & retention product can not be obtained.
where elimination competes substitution, it shows inversion & retention product can not be obtained.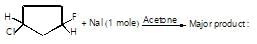
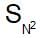 reactions involve inversion of configuration,as given compound has ‘R’ configuration, so product will be ‘S’
reactions involve inversion of configuration,as given compound has ‘R’ configuration, so product will be ‘S’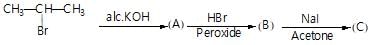
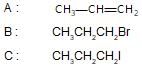
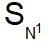 involves partial racemisation while
involves partial racemisation while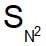 involves Walden inversion
involves Walden inversion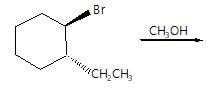
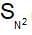 mechanism
mechanism














