Hydrocarbons - Practice Test (1) - Class 9 MCQ
25 Questions MCQ Test - Hydrocarbons - Practice Test (1)
The reaction given below is used to produce polythene. This is an example of _______ reaction

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
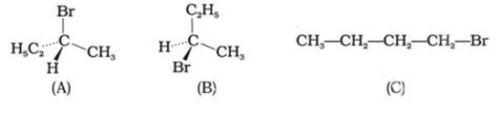
The addition of HBr to 1-butene gives a mixture of products A, B and C. The mixture consists of
The synthesis of 3-octyne is achieved by adding a bromoalkane into a mixture of sodium amide and an alkyne. The bromoalkane and alkyne respectively are
Phenyl magnesium bromide reacts with methanol to give
Which of the following will not show geometrical isomerism?
One mole of a symmetrical alkene on ozonolysis gives two moles of an aldehyde having a molecular mass of 44 u. The alkene is
A gas decolourised by KMnO4 solution but gives no precipitate with ammoniacal cuprous chloride is
Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
n-propyl bromide on treating with alcoholic KOH produces
What would be the product formed when 1-Bromo-3-chlorocyclobutane reacts with two equivalents of metallic sodium in ether?
(A)
(B)
(C)
(D)
Which of these is not a characteristic of aromatic Hydrocarbons?
In the preparation of alkynes from vicinal dihalides, the reaction is
Arrange the following carbanions in order of their decreasing stability.
(A) H3C – C ≡≡C–
(B) H – C ≡ C–
(C) H3C−−−CH2−−H3C−−−CH2−−
2 methylbutane on reacting with bromine in the presence of sunlight gives mainly
Majority of the reactions of alkynes are the examples of
Arrange the following alkyl halides in decreasing order of the rate of β−−β−− elimination reaction with alcoholic KOH
In the following sequence of reactions, the alkene affords the compound 'B'
The compound B is
Alkyl halides react with dialkyl copper reagents to give

















