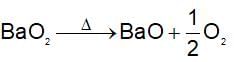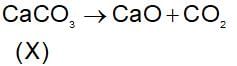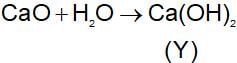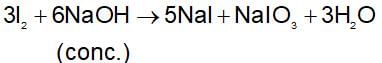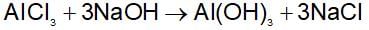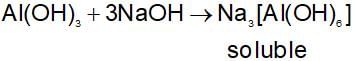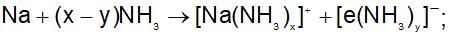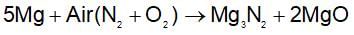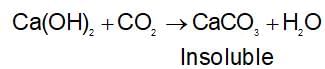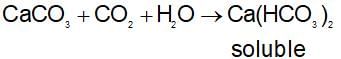Hydrogen & S-Block - Class 12 MCQ
30 Questions MCQ Test - Hydrogen & S-Block
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A solid compound ‘X’ on heating gives CO2 gas and a residue. The residue mixed with water forms ‘Y’ on passing an excess of CO2 through ‘Y’ in water, a clear solution ‘Z’ is obtained. On boiling ‘Z’ compound ‘X’ is reformed. The compound ‘X’ is:
Beryllium shows diagonal relationship with aluminium. Which of the following similaeity is incorrect?
A metal M readily forms sulphate MSO4 which is water insoluble. It forms oxide MO which becomes inert on heating. It forms insoluble hydroxide which is soluble in NaOH. The metal M is:
An inorganic compound liberates O2 when heated, turns an acid solution of KI brown and reduces acidified KMnO4. The substance is:
When zeolites, (hydrated sodium aluminium silicate) is treated with hard water, the sodium ions are exchange with :
The principal products obtained on heating iodine with concentrated caustic soda solution are:
A colourless salt gives violet colour to Bunsen flame and also turns moistured litmus paper blue. It is:
What would you observe if excess of dilute NaOH solution is added and shaken with an aqueous solution of aluminum chloride?
When sodium metal is dissolved in liquid ammonia, a blue solution is formed. The blue colour is due to:
An aqueous solution of salt of sodium (NaX) on boiling with MgCI2 gives white precipitate, hence anion X is:
When magnesium is burnt in air, compounds of magnesium formed are magnesium oxide and:
Disodium hydrogen phosphate in presence of NH4CI and NH4OH gives a white ppt. with a solution of Mg2+ ion. The precipitate is:
If CO2 is passed in excess into lime water, the milkiness first formed disapperars due to: