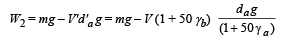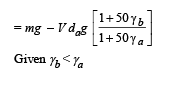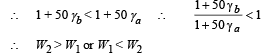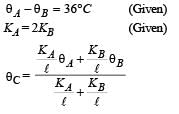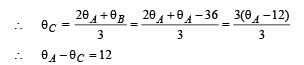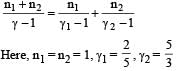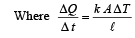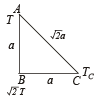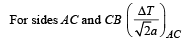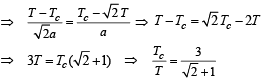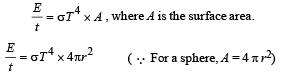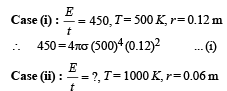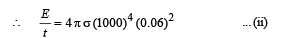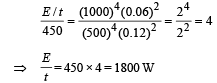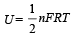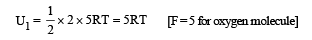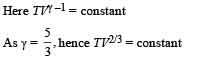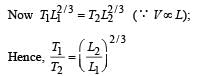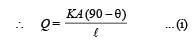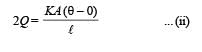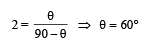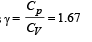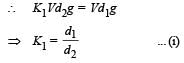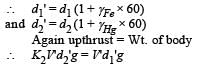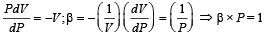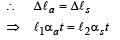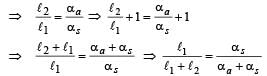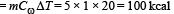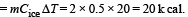JEE Advanced (Single Correct MCQs): Heat & Thermodynamics - JEE MCQ
30 Questions MCQ Test - JEE Advanced (Single Correct MCQs): Heat & Thermodynamics
A constant volume gas thermometer works on (1980)
A metal ball immersed in alcohol weighs W1 at 0°C and W2 at 50°C. The coefficient of expansion of cubical the metal is less than that of the alcohal. Assuming that the density of the metal is large compared to that of alcohol, it can be shown that
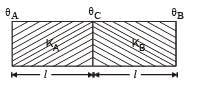
A wall has two layers A and B, each made of different material. Both the layers have the same thickness. The thermal conductivity of the meterial of A is twice that of B.Under thermal equilibrium, the temperature difference across the wall is 36°C. The temperature difference across the layer A is
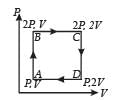
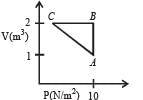
An ideal monatomic gas is taken round the cycle ABCDA as shown in the P – V diagram (see Fig.). The work done during the cycle is
If one mole of a monatomic gas is mixed with one mole of a diatomic gas
, the value of γ for mixture is
From the following statements concerning ideal gas at any given temperature T, select the correct one(s)
Three rods of identical cross-sectional area and made from the same metal from the sides of an isosceles traingle ABC, right-angled at B. The points A and B are maintained at temperatures T and (√2) T respectively. In the steady state, the temperature of the point C is Tc. Assuming that only heat conduction takes place, Tc / T is
Two metallic spheres S1 and S2 are made of the same material and have got identical surface finish. The mass of S1 is thrice that of S2. Both the spheres are heated to the same high temperature and placed in the same room having lower temperature but are thermally insulated from each other. The ratio of the initial rate of cooling of S1 to that of S2 is
The average translational kinetic energy of O2 (relative molar mass 32) molecules at a particular temperature is 0.048 eV.
The translational kinetic energy of N2 (relative molar mass 28) molecules in eV at the same temperature is
A vessel contains 1 mole of O2 gas (relative molar mass 32) at a temperature T. The pressure of the gas is P. An identical vessel containing one mole of He gas (relative molar mass 4) at a temperature 2T has a pressure of
A spherical black body with a radius of 12 cm radiates 450 W power at 500 K. if the radius were halved and the temperature doubled, the power radiated in watt would be
A closed compartment containing gas is moving with some acceleration in horizontal direction. Neglect effect of gravity.Then the pressure in the compartment is
A gas mixture consists of 2 moles of oxygen and 4 moles of argon at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
The ratio of the speed of sound in nitrogen gas to that in helium gas, at 300 K is
A monatomic ideal gas, initially at temperature T1, is enclosed in a cylinder fitted with a frictionless piston. The gas is allowed to expand adiabatically to a temperature T2 by releasing the piston suddenly. If L1 and L2 are the length of the gas column before and after expansion respectively,
then is given by
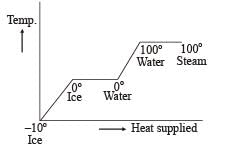
A block of ice at –10°C is slowly heated and converted to steam at 100°C. Which of the following curves represents the phenomenon qualitatively ?
An ideal gas is initially at temperature T and volume V. Its volume is increased by ΔV due to an increase in temperature ΔT, pressure remaining constant. The quantity
varies with temperature as
Starting with the same initial conditions, an ideal gas expands from volume V1 to V2 in three different ways. The work done by the gas is W1 if the process is purely isothermal, W2 if purely isobaric and W3 if purely adiabatic. Then
The plots of intensity versus wavelength for three black bodies at temperature T1, T2 and T3 respectively are as shown. Their temperatures are such that
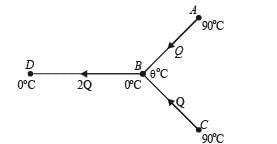
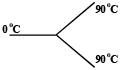
Three rods made of same material and having the same cross-section have been joined as shown in the figure. Each rod is of the same length. The left and right ends are kept at 0oC and 90oC respectively. The temperature of the junction of the three rods will be
In a given process on an ideal gas, dW = 0 and dQ < 0. Then for the gas
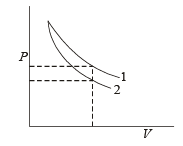
P-V plots for two gases dur ing adiabatic pr ocesses are shown in the figure. Plots 1 and 2 should correspond respectively to
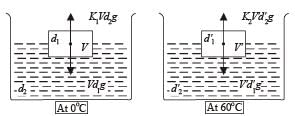
When a block of iron floats in mercury at 0oC, fraction k1 of its volume is submerged, while at the temperature 60oC, a fraction k2 is seen to be submerged. If the coefficient of volume expansion of iron is γFe and that of mercury is γHg, then the ratio k1/k2 can be expressed as
An ideal gas is taken through the cycle as shown in the figure. If the net heat supplied to the gas in the cycle is 5J, the work done by the gas in the process C⇒A is
Which of the followin g graphs cor rectly represents the variation of 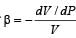 with P for an ideal gas at constant temperature ?
with P for an ideal gas at constant temperature ?
An ideal Black-body at room temperature is thrown into a furnace. It is observed that
The graph, shown in the adjacent diagram, represents the variation of temperature (T) of two bodies, x and y having same surface area, with time (t) due to the emission of radiation. Find the correct relation between the emissivity and absorptivity power of the two bodies
Two rods, one of aluminum and the other made of steel, having initial length l1 and l2 are connected together to form a single rod of length l1 + l2. The coefficients of linear expansion for aluminum and steel are αa and αs and respectively. If the length of each rod increases by the same amount when their temperature are raised by t0C, then find the ratiol1/(l1 + l2)
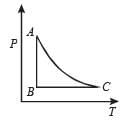
The PT diagram for an ideal gas is shown in the figure, where AC is an adiabatic process, find the corresponding PV diagram.
2 kg of ice at –200C is mixed with 5kg of water at 200C in an insulating vessel having a negligible heat capacity. Calculate the final mass of water remaining in the container. It is given that the specific heats of water & ice are 1kcal/kg/0C & 0.5 kcal/kg/0C while the latent heat of fusion of ice is 80 kcal/kg