MCQ (Practice) - Isomerism (Level 2) - Class 11 MCQ
25 Questions MCQ Test - MCQ (Practice) - Isomerism (Level 2)
A pure simple of 2-chlorobutane shows rotation of PPL by 30º in standard conditions. When above sample is made impure by mixing its opposite form, so that the composition of the mixture become 87.5% d-form and 12.5% l-form, then what will be the observed rotation for the mixture.
When an optically active compound is placed in a 10 dm tube is present 20 gm in a 200 ml solution rotates the PPL by 30º. Calculate the angle of rotation & specific angle of rotation if above solution diluted to 1 Litre.
In the given following compound find out the pair of enantiomers and diastereomers
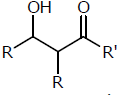
The molecule (s) that exist as meso structure(s)
Among the following, a pair of resolvable configurational enantiomers is given by
Which two of the following compounds are identical ?
Which two of the following compounds are diasteromers ?
Which of the following is properly classified as a meso compound ?
Which two of the following compounds represents a pair of enantiomers ?
The drawing on the rigth shows that trans-1, 3-dichlorocyclohexane is chiral. Efforts to resolve this compound fail. Why ?
How many stereoisomers of (CH3)2CHCH = CHCH2CH(OH)CH2Br are possible ?
What common symmetry of elements if any are found in the stable chair conformer of trans-1, 2- dichlorocyclohexane ?
How many stereoisomer are possible for the following molecule ?
How many stereoisomers are possible for the following compound ?
Optical rotation produced byis 36° then that product by
Dextrorotatory α-pinene has a specific rotaiton [α]D20 = +51.3°. A sample of α-pinene containing both the enantiomers was found to have a specific rotationa value [α]D20 = +30.8°. The percentages of the (+) and (–) enantiomers present in the sample are, respectively.
(+)-mandelic acid has a specific rotation of 158°. What would be the observed specific rotation of a mixture of 25% (–)-mandelic acid and 75% (+)-mandelic acid ?
Number of structural isomers of compound having molecular formula C4H7Cl.
Which of the following sugars has the configuration (2S 3R, 4R) ?
Which of the following statements must be true for two pure chiral isomers ?
Which of the following statements is true for a pair of diastereomers ?
The isomerism exhibited by acetic acid and methyl formate is
Which of the following compound will exhibit Cis-trans (geometrical) isomerism ?
Molecular formular C3H6Br2 can have (including stereoisomers):














