MCQ (Previous Year Questions) - Chemical Equilibrium (Level 2) - JEE MCQ
7 Questions MCQ Test - MCQ (Previous Year Questions) - Chemical Equilibrium (Level 2)
When two reactans A and B are mixed to give products C and D, the reaction quotient Q, at the initial stages of the reaction : [JEE 2000]
For the reversible reactions :
N2(g) + 3 H2(g)  2NH3(g) at 500°C. The value of Kp is 1.44 × 10-5, when partial pressure is measured in atmospheres. The corresponding value of Kc with concentration in mol L-1 is : [JEE 2000]
2NH3(g) at 500°C. The value of Kp is 1.44 × 10-5, when partial pressure is measured in atmospheres. The corresponding value of Kc with concentration in mol L-1 is : [JEE 2000]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
At constant temperature, the equilibrium constant (Kp) for the decomposition reaction. N2O4  2NO2 is expressed by Kp=
2NO2 is expressed by Kp= 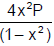 where P is pressure, x is extent of decomposition. Which of the following statement is true ? [JEE 2001]
where P is pressure, x is extent of decomposition. Which of the following statement is true ? [JEE 2001]
Consider the following equilibrium in a closed container : N2O4(g) 2NO2(g). [JEE 2002]
At a fixed temperature, the volume of the reaction container is halved. For this change, which of the following statements holds true regarding the equilibrium constant (KP) and degree of dissociation (α) :
If Ag+ + NH3 [Ag(NH3)]+ ; K1=1.6 × 103 and
[Ag(NH3)+] + NH3[Ag(NH 3)+] ; K2=6.8 × 103
The formation constant of [Ag(NH3)2]+ is : [JEE 2006]
The equilibrium
2CuI Cuº + CuII
in aqueous medium at 25ºC shifts towards the left in the presence of [JEE 2011]
The thermal dissociation equilibrium of CaCO3 (s) is studied under different conditions.
CaCO3 (s) CaO(s) + CO2(g)
For this equilibrium, the correct statement(s) is (are) [JEE 2013]

















