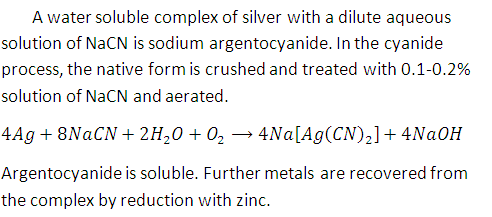MCQ (Previous Year Questions) - Isolation (Level 2) - JEE MCQ
20 Questions MCQ Test - MCQ (Previous Year Questions) - Isolation (Level 2)
In the commerical electrochemical process for aluminium extraction, the electrolyte used as : [JEE 1999]
The chemical process in the production of steel from haematite ore involve: [JEE 2000 Qualifying]
Electrolytic reduction of alumina to aluminium by Hall–Heroult process is carried out : [JEE 2000 Qualifying]
The chemical composition of "slag" formed during the smelting process in the extraction of copper is : [JEE 2001 Qualifying]
Which of the follwoing processes is used in extractive metallurgy of magnesium? [JEE 2002 Qualifying]
In the process of extraction of gold, Roasted gold ore + CN_ + H2O [X] + OH_ [X] + Zn ¾→ [Y] + Au Identify the complexes [X] and [Y] : [JEE 2003 Qualifying]
The methods chiefly used for the extraction of lead and tin from their ores are respectively : [JEE 2004]
Which ore contains both iron and copper? [JEE 2004]
Extraction for zinc from zinc blende is achieved by : [JEE 2007]
Native silver metal forms a water soluble complex with a dilute aqueous solution of NaCl in the presence of : [JEE 2008]
Copper is the most noble of the first row transition metals and occurs in small deposits in several countries. Ores of copper include chalcanthite (CuSO4. 5H2O) atacamite (Cu2Cl(OH)3), cuprite (Cu2O), copper glance (Cu2S) and malachite (Cu2(OH)2CO3). However, 80% of the world copper production comes from the ore chalcopyrite (CuFeS2). The extraction of copper from chalcopyrite involves partial roasting, revoval of iron and self -reduction. [JEE 2010]
Partial roasting of chalcopyrite produces
Copper is the most noble of the first row transition metals and occurs in small deposits in several countries. Ores of copper include chalcanthite (CuSO4. 5H2O) atacamite (Cu2Cl(OH)3), cuprite (Cu2O), copper glance (Cu2S) and malachite (Cu2(OH)2CO3). However, 80% of the world copper production comes from the ore chalcopyrite (CuFeS2). The extraction of copper from chalcopyrite involves partial roasting, revoval of iron and self -reduction. [JEE 2010]
Iron is removed from chalcopyrite as
Copper is the most noble of the first row transition metals and occurs in small deposits in several countries. Ores of copper include chalcanthite (CuSO4. 5H2O) atacamite (Cu2Cl(OH)3), cuprite (Cu2O), copper glance (Cu2S) and malachite (Cu2(OH)2CO3). However, 80% of the world copper production comes from the ore chalcopyrite (CuFeS2). The extraction of copper from chalcopyrite involves partial roasting, revoval of iron and self -reduction. [JEE 2010]
In self -reduction, the reducing species is
Oxidation states of the metal in the minerals haematite and magnetite, respectively, are [JEE 2011]
Extraction of metal from the ore cassiterite involves [JEE 2011]
Match the extraction processes listed in column_I with metals listed in column–II. [JEE 2006]
Column–I Column–II
(A) Self reduction (P) Lead
(B) Carbon reduction (Q) Silver
(C) Complex formation and displacement by metal (R) Copper
(D) Decomposition of iodide (S) Boron
Match the conversions in Column–I with the type(s) of reaction(s) given in Column–II. Indicate your answer by darkening the appropriate bubbles of the 4 × 4 matrix given in the ORS. [JEE 2008]
Column–I Column–II
(A) PbS ¾→ PbO (P) Roasing
(B) CaCO3 ¾→ CaO (Q) Calcination
(C) ZnS ¾→ Zn (R) Carbon reduction
(D) Cu2S ¾→ Cu (S) Self reduction
In extractive metallurgy of zinc partial fusion of ZnO with coke is called _________ and reduction of the ore to the molten metal is called __________ (smelting, calcining, roasting, sintering) [JEE 1988]
In the cyanide extraction process of silver from argentite ore, the oxidizing and reducing agent used are [JEE 2012]
Sulfide ores are common ofr the metals [JEE 2013]